当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Step-Economic Synthesis of Biomimetic β-Ketopolyene Thioesters and Demonstration of Their Usefulness in Enzymatic Biosynthesis Studies.
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01348 Johannes Wunderlich 1 , Theresa Roß 1 , Marius Schröder 1 , Frank Hahn 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01348 Johannes Wunderlich 1 , Theresa Roß 1 , Marius Schröder 1 , Frank Hahn 1
Affiliation
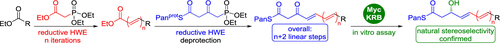
|
Studies on the biosynthetic processing of polyene thioester intermediates are complicated by limited access to appropriate substrate surrogates. We present a step-economic synthetic access to biomimetic β-ketopolyene thioesters that is based on an Ir-catalyzed reductive Horner–Wadsworth–Emmons olefination. New β-ketotriene and pentaenethioates of pantetheine and N-acetylcysteamine were exemplarily synthesized via short and concise routes. The usefulness of these compounds was demonstrated in an in vitro assay with the ketoreductase domain MycKRB from mycolactone biosynthesis.
中文翻译:

仿生β-酮基多烯硫酸酯的逐步经济合成及其在酶促生物合成研究中的用途。
多烯硫酯中间体的生物合成工艺研究由于难以获得合适的底物替代物而变得复杂。我们提出了一种仿生的β-酮多烯硫酯的逐步经济合成方法,该方法基于Ir催化的还原性Horner-Wadsworth-Emmons烯烃化反应。示例性地通过简短的途径合成泛酸的新的β-酮三烯和戊烯硫醚以及N-乙酰基半胱胺。这些化合物的有用性在体外测定中得到了证明,该酮还原酶结构域来自Mycolactone生物合成的MycKRB。
更新日期:2020-07-02
中文翻译:

仿生β-酮基多烯硫酸酯的逐步经济合成及其在酶促生物合成研究中的用途。
多烯硫酯中间体的生物合成工艺研究由于难以获得合适的底物替代物而变得复杂。我们提出了一种仿生的β-酮多烯硫酯的逐步经济合成方法,该方法基于Ir催化的还原性Horner-Wadsworth-Emmons烯烃化反应。示例性地通过简短的途径合成泛酸的新的β-酮三烯和戊烯硫醚以及N-乙酰基半胱胺。这些化合物的有用性在体外测定中得到了证明,该酮还原酶结构域来自Mycolactone生物合成的MycKRB。











































 京公网安备 11010802027423号
京公网安备 11010802027423号