当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium(III)-Catalyzed Cyclopropane C-H/C-C Activation Sequence Provides Diastereoselective Access to α-Alkoxylated γ-Lactams.
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01606 Benoit Audic 1 , Nicolai Cramer 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01606 Benoit Audic 1 , Nicolai Cramer 1
Affiliation
|
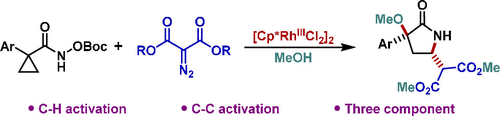
A Cp*Rh(III)-catalyzed C–H/C–C bond activation sequence of cyclopropyl hydroxamates has been developed. The three-component process allows trapping of the intermediate rhodacycle with diazomalonates and an alcohol nucleophile to provide access to synthetically valuable α-alkoxylated γ-lactams with trans diastereoselectivity.
中文翻译:

铑(III)催化的环丙烷CH / CC活化序列可提供非对映选择性地进入α-烷氧基化的γ-内酰胺。
已经开发出了Cp * Rh(III)催化的环丙基异羟肟酸酯的C–H / C–C键激活序列。三组分过程允许用重氮丙二酸酯和醇亲核试剂捕获中间的铑环,从而提供具有反式非对映选择性的合成有价值的α-烷氧基化γ-内酰胺。
更新日期:2020-07-02
中文翻译:

铑(III)催化的环丙烷CH / CC活化序列可提供非对映选择性地进入α-烷氧基化的γ-内酰胺。
已经开发出了Cp * Rh(III)催化的环丙基异羟肟酸酯的C–H / C–C键激活序列。三组分过程允许用重氮丙二酸酯和醇亲核试剂捕获中间的铑环,从而提供具有反式非对映选择性的合成有价值的α-烷氧基化γ-内酰胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号