当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Acyloin Rearrangements of α-Ketols, α-Hydroxy Aldehydes, and α-Iminols by N,N'-Dioxide-Metal Complexes.
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01626 Li Dai 1 , Xiangqiang Li 1 , Zi Zeng 1 , Shunxi Dong 1 , Yuqiao Zhou 1 , Xiaohua Liu 1 , Xiaoming Feng 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01626 Li Dai 1 , Xiangqiang Li 1 , Zi Zeng 1 , Shunxi Dong 1 , Yuqiao Zhou 1 , Xiaohua Liu 1 , Xiaoming Feng 1
Affiliation
|
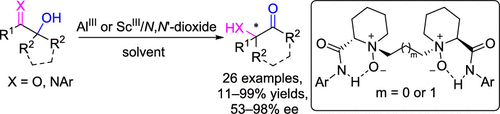
A highly enantioselective acyloin rearrangement of cyclic α-ketols has been developed with a chiral Al(III)–N,N′-dioxide complex as catalyst. This strategy provided an array of optically active 2-acyl-2-hydroxy cyclohexanones in moderate to good yields with high enantioselectivities. The asymmetric isomerizations of acyclic α-hydroxy aldehydes and α-iminols were achieved as well under modified conditions, affording the corresponding chiral α-hydroxy ketones and α-amino ketones in moderate results. Moreover, further transformations of product to enantioenriched diols were carried out.
中文翻译:

N,N'-二氧化物-金属络合物催化α-酮醇,α-羟基醛和α-氨基缩醛的不对称酰基环重排。
环状α-酮醇的高度对映选择性酰基肌醇重排反应已经开发出来,其中手性Al(III)–N,N'-二氧化物配合物为催化剂。该策略以中等到良好的产率和高对映选择性提供了一系列光学活性的2-酰基-2-羟基环己酮。在改性条件下也可以实现无环α-羟基醛和α-亚氨基的不对称异构化,得到中等程度的相应手性α-羟基酮和α-氨基酮。此外,将产物进一步转化为对映体富集的二醇。
更新日期:2020-07-02
中文翻译:

N,N'-二氧化物-金属络合物催化α-酮醇,α-羟基醛和α-氨基缩醛的不对称酰基环重排。
环状α-酮醇的高度对映选择性酰基肌醇重排反应已经开发出来,其中手性Al(III)–N,N'-二氧化物配合物为催化剂。该策略以中等到良好的产率和高对映选择性提供了一系列光学活性的2-酰基-2-羟基环己酮。在改性条件下也可以实现无环α-羟基醛和α-亚氨基的不对称异构化,得到中等程度的相应手性α-羟基酮和α-氨基酮。此外,将产物进一步转化为对映体富集的二醇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号