当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
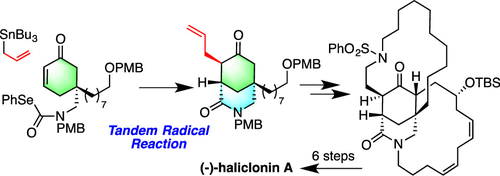
Formal Synthesis of (-)-Haliclonin A: Stereoselective Construction of an Azabicyclo[3.3.1]nonane Ring System by a Tandem Radical Reaction.
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01627 Keita Komine 1 , Yasuhiro Urayama 1 , Taku Hosaka 1 , Yuki Yamashita 1 , Hayato Fukuda 1 , Susumi Hatakeyama 2 , Jun Ishihara 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01627 Keita Komine 1 , Yasuhiro Urayama 1 , Taku Hosaka 1 , Yuki Yamashita 1 , Hayato Fukuda 1 , Susumi Hatakeyama 2 , Jun Ishihara 1
Affiliation
|
A formal synthesis of (−)-haliclonin A, isolated from the marine sponge Haliclona sp. in Korea, is described. The key feature of the synthesis includes the highly stereoselective tandem radical reaction to construct the azabicyclo[3.3.1]nonane core and the enantioselective formation of an all-carbon quaternary center via the Pd-mediated deracemization.
中文翻译:

(-)-Haliclonin A的形式合成:通过串联自由基反应立体构筑氮杂双[3.3.1]壬烷环系统。
从海洋海绵Haliclona sp。分离出的(-)-haliclonin A的正式合成。在韩国描述。合成的关键特征包括高度立体选择性的串联自由基反应,以构建氮杂双环[3.3.1]壬烷核,以及通过Pd介导的脱碳反应形成全碳四元中心的对映选择性。
更新日期:2020-07-02
中文翻译:

(-)-Haliclonin A的形式合成:通过串联自由基反应立体构筑氮杂双[3.3.1]壬烷环系统。
从海洋海绵Haliclona sp。分离出的(-)-haliclonin A的正式合成。在韩国描述。合成的关键特征包括高度立体选择性的串联自由基反应,以构建氮杂双环[3.3.1]壬烷核,以及通过Pd介导的脱碳反应形成全碳四元中心的对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号