当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective, Multicomponent Approach to Quaternary Substituted Hydroindole Scaffolds.
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01650 Nicholas P Massaro 1, 2 , Joshua G Pierce 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01650 Nicholas P Massaro 1, 2 , Joshua G Pierce 1, 2
Affiliation
|
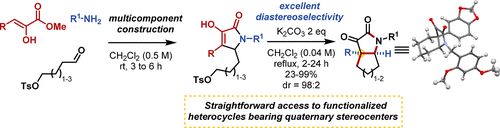
The Amaryllidaceae alkaloids have been a target of synthesis for decades due to their complex architectures and biological activity. A central feature of these natural product cores is a quaternary substituted hydroindole heterocycle. Building off the foundation of our previous multicomponent approach to highly functionalized pyrrolidinones, herein we report a highly convergent, diastereoselective, multicomponent approach to access the hydroindole cores present within crinine, haemanthamine, pretazettine, and various other bioactive alkaloids. These scaffolds are additionally useful as building blocks for druglike molecules and natural product like library generation.
中文翻译:

立体选择性,多组分方法取代季铵盐的氢化吲哚骨架。
数十年来,金莲花科生物碱一直是合成的目标,因为它们复杂的结构和生物活性。这些天然产物核心的主要特征是季取代的氢吲哚杂环。在我们先前对高度官能化的吡咯烷酮的多组分方法的基础之上,本文报道了一种高度收敛,非对映选择性的多组分方法,可用于获得存在于可丽宁,甘露糖胺,普他他汀和各种其他生物活性生物碱中的氢化吲哚核。这些支架还可用作药物样分子和天然产物(如文库生成)的构建基块。
更新日期:2020-07-02
中文翻译:

立体选择性,多组分方法取代季铵盐的氢化吲哚骨架。
数十年来,金莲花科生物碱一直是合成的目标,因为它们复杂的结构和生物活性。这些天然产物核心的主要特征是季取代的氢吲哚杂环。在我们先前对高度官能化的吡咯烷酮的多组分方法的基础之上,本文报道了一种高度收敛,非对映选择性的多组分方法,可用于获得存在于可丽宁,甘露糖胺,普他他汀和各种其他生物活性生物碱中的氢化吲哚核。这些支架还可用作药物样分子和天然产物(如文库生成)的构建基块。











































 京公网安备 11010802027423号
京公网安备 11010802027423号