当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-Catalyzed Ring-Opening Hydroacylation of Alkylidenecyclopropanes with Chelating Aldehydes for the Synthesis of γ,δ-Unsaturated Ketones.
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01751 Hong-Shuang Li 1 , Shi-Chao Lu 2 , Zhi-Xin Chang 1 , Liqiang Hao 1 , Fu-Rong Li 1 , Chengcai Xia 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01751 Hong-Shuang Li 1 , Shi-Chao Lu 2 , Zhi-Xin Chang 1 , Liqiang Hao 1 , Fu-Rong Li 1 , Chengcai Xia 1
Affiliation
|
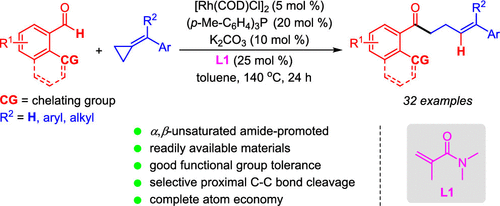
The first intermolecular ring-opening hydroacylation of alkylidenecyclopropanes with chelating aldehydes through a rhodium-catalyzed acrylamide-promoted protocol is reported. This highly efficient catalytic system enables the direct synthesis of a diverse range of linear γ,δ-unsaturated ketones. Good functional group compatibility is demonstrated for the completely atom-economical and remarkably selective proximal C–C bond cleavage process. Mechanistic studies reveal that the bidentate coordination of N,N-dimethylmethacrylamide (L1) to the acylrhodium intermediates might facilitate the cyclopropane ring fragmentation and isomerization.
中文翻译:

铑催化的亚烷基环丙烷与螯合醛的开环加氢酰化反应,合成γ,δ-不饱和酮。
据报道,通过铑催化的丙烯酰胺促进方案,亚烷基环丙烷与螯合醛的首次分子间开环加氢酰化反应。这种高效的催化系统可直接合成各种线性的γ,δ-不饱和酮。良好的官能团相容性在完全原子经济且选择性强的近端C–C键裂解过程中得到证明。机理研究表明,N,N-二甲基甲基丙烯酰胺(L1)与酰基铑中间体的双齿配位可能促进环丙烷环的断裂和异构化。
更新日期:2020-07-02
中文翻译:

铑催化的亚烷基环丙烷与螯合醛的开环加氢酰化反应,合成γ,δ-不饱和酮。
据报道,通过铑催化的丙烯酰胺促进方案,亚烷基环丙烷与螯合醛的首次分子间开环加氢酰化反应。这种高效的催化系统可直接合成各种线性的γ,δ-不饱和酮。良好的官能团相容性在完全原子经济且选择性强的近端C–C键裂解过程中得到证明。机理研究表明,N,N-二甲基甲基丙烯酰胺(L1)与酰基铑中间体的双齿配位可能促进环丙烷环的断裂和异构化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号