当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Stereoselective Hydrodefluorination of Tetrasubstituted gem-Difluoroalkenes.
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01813 Qiao Ma 1 , Caroline Liu 1 , Gavin Chit Tsui 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01813 Qiao Ma 1 , Caroline Liu 1 , Gavin Chit Tsui 1
Affiliation
|
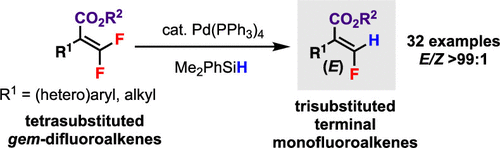
A highly stereoselective palladium(0)-catalyzed hydrodefluorination (HDF) of tetrasubstituted gem-difluoroalkenes is developed. By using catalytic Pd(PPh3)4 (2.5–5 mol %) and hydrosilane Me2PhSiH, various trisubstituted terminal (E)-monofluoroalkenes can be synthesized with excellent E/Z selectivity (>99:1) and good functional group tolerability. The key stereocontrol should be exerted by an ester-directed C–F bond oxidative addition step in the catalytic cycle.
中文翻译:

四取代的宝石-二氟烯烃的钯催化立体选择性加氢脱氟。
开发了四取代的宝石-二氟烯烃的高度立体选择性的钯(0)催化加氢脱氟(HDF)。通过使用催化性Pd(PPh 3)4(2.5-5 mol%)和氢硅烷Me 2 PhSiH,可以合成各种三取代端基(E)-单氟烯烃,具有出色的E / Z选择性(> 99:1)和良好的官能团耐受性。关键的立体控制应通过催化循环中酯基的CF键氧化加成步骤来实现。
更新日期:2020-07-02
中文翻译:

四取代的宝石-二氟烯烃的钯催化立体选择性加氢脱氟。
开发了四取代的宝石-二氟烯烃的高度立体选择性的钯(0)催化加氢脱氟(HDF)。通过使用催化性Pd(PPh 3)4(2.5-5 mol%)和氢硅烷Me 2 PhSiH,可以合成各种三取代端基(E)-单氟烯烃,具有出色的E / Z选择性(> 99:1)和良好的官能团耐受性。关键的立体控制应通过催化循环中酯基的CF键氧化加成步骤来实现。









































 京公网安备 11010802027423号
京公网安备 11010802027423号