当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis Provides Strong Evidence: Xestocyclamine A is the Enantiomer of Ingenamine
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-16 , DOI: 10.1021/jacs.0c05347 Zhanchao Meng 1 , Alois Fürstner 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-16 , DOI: 10.1021/jacs.0c05347 Zhanchao Meng 1 , Alois Fürstner 1
Affiliation
|
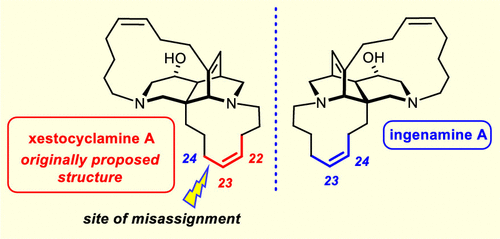
Xestocyclamine A ((−)-1) is featured prominently in a biosynthesis pathway leading to a large family of polycyclic alkaloids. The first total synthesis now proves that the structure of this compound had originally been misassigned. The route to (−)-1 is based on a double Michael addition for the formation of the bridged diazadecalin core and a palladium-catalyzed decarboxylative allylation to install the quaternary bridgehead center. Ring-closing alkyne metathesis allowed a 13-membered cycloalkyne to be forged, which was selectively reduced during an involved sequence of hydroboration/selective protodeborylation/alkyl-Suzuki coupling used to close the 11-membered ring. Crystallographic data prove the identity of synthetic (−)-1 with nominal xestocyclamine, but the spectra differ from those of the authentic alkaloid. To clarify the point, the synthesis was redirected toward ingenamine (3), which is supposedly a positional isomer of 1. The recorded data confirm the assignment of this particular natural product and strongly suggest that xestocyclamine A is in fact the enantiomer of ingenamine (+)-3.
中文翻译:

全合成提供强有力的证据:Xestocyclamine A 是 Ingenamine 的对映异构体
Xestocyclamine A ((-)-1) 在导致一大类多环生物碱的生物合成途径中占有突出地位。现在第一次全合成证明该化合物的结构最初被错误分配。到 (-)-1 的路线基于双迈克尔加成以形成桥接二氮杂萘烷核心和钯催化脱羧烯丙基化以安装四元桥头中心。闭环炔烃复分解允许锻造 13 元环炔烃,在用于闭合 11 元环的硼氢化/选择性原脱硼酸化/烷基-铃木偶联的相关序列中选择性还原。晶体学数据证明合成 (-)-1 与标称的 xestocyclamine 相同,但光谱与真实生物碱的光谱不同。为了澄清这一点,
更新日期:2020-06-16
中文翻译:

全合成提供强有力的证据:Xestocyclamine A 是 Ingenamine 的对映异构体
Xestocyclamine A ((-)-1) 在导致一大类多环生物碱的生物合成途径中占有突出地位。现在第一次全合成证明该化合物的结构最初被错误分配。到 (-)-1 的路线基于双迈克尔加成以形成桥接二氮杂萘烷核心和钯催化脱羧烯丙基化以安装四元桥头中心。闭环炔烃复分解允许锻造 13 元环炔烃,在用于闭合 11 元环的硼氢化/选择性原脱硼酸化/烷基-铃木偶联的相关序列中选择性还原。晶体学数据证明合成 (-)-1 与标称的 xestocyclamine 相同,但光谱与真实生物碱的光谱不同。为了澄清这一点,











































 京公网安备 11010802027423号
京公网安备 11010802027423号