当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Dynamics of N-Glycosylated Human Ribonuclease 1.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.biochem.0c00191 Henry R Kilgore 1 , Andrew P Latham 1 , Valerie T Ressler 1 , Bin Zhang 1 , Ronald T Raines 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.biochem.0c00191 Henry R Kilgore 1 , Andrew P Latham 1 , Valerie T Ressler 1 , Bin Zhang 1 , Ronald T Raines 1
Affiliation
|
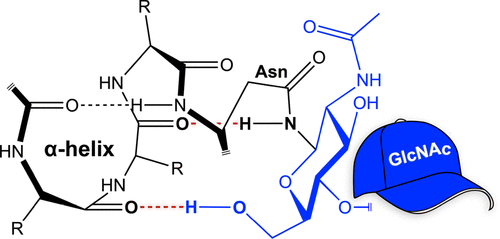
Glycosylation is a common modification that can endow proteins with altered physical and biological properties. Ribonuclease 1 (RNase 1), which is the human homologue of the archetypal enzyme RNase A, undergoes N-linked glycosylation at asparagine residues 34, 76, and 88. We have produced the three individual glycoforms that display the core heptasaccharide, Man5GlcNAc2, and analyzed the structure of each glycoform by using small-angle X-ray scattering along with molecular dynamics simulations. The glycan on Asn34 is relatively compact and rigid, donates hydrogen bonds that “cap” the carbonyl groups at the C-terminus of an α-helix, and enhances protein thermostability. In contrast, the glycan on Asn88 is flexible and can even enter the enzymic active site, hindering catalysis. The N-glycosylation of Asn76 has less pronounced consequences. These data highlight the diverse behaviors of Man5GlcNAc2 pendants and provide a structural underpinning to the functional consequences of protein glycosylation.
中文翻译:

N-糖基化人核糖核酸酶的结构和动力学1。
糖基化是一种常见的修饰,可以赋予蛋白质改变的物理和生物学特性。核糖核酸酶1(RNase 1)是原型酶RNase A的人类同源物,在天冬酰胺残基34、76和88处经历N联糖基化作用。我们产生了展示核心七糖的三种独立糖型,Man 5 GlcNAc 2,并通过小角度X射线散射和分子动力学模拟分析了每种糖型的结构。Asn34上的聚糖相对紧凑且刚性强,提供氢键“封盖”α螺旋C端的羰基,并增强了蛋白质的热稳定性。相反,Asn88上的聚糖具有柔韧性,甚至可以进入酶活性位点,从而阻碍了催化作用。Asn76的N-糖基化作用不太明显。这些数据突出了Man 5 GlcNAc 2链坠的不同行为,并提供了蛋白质糖基化功能后果的结构基础。
更新日期:2020-06-16
中文翻译:

N-糖基化人核糖核酸酶的结构和动力学1。
糖基化是一种常见的修饰,可以赋予蛋白质改变的物理和生物学特性。核糖核酸酶1(RNase 1)是原型酶RNase A的人类同源物,在天冬酰胺残基34、76和88处经历N联糖基化作用。我们产生了展示核心七糖的三种独立糖型,Man 5 GlcNAc 2,并通过小角度X射线散射和分子动力学模拟分析了每种糖型的结构。Asn34上的聚糖相对紧凑且刚性强,提供氢键“封盖”α螺旋C端的羰基,并增强了蛋白质的热稳定性。相反,Asn88上的聚糖具有柔韧性,甚至可以进入酶活性位点,从而阻碍了催化作用。Asn76的N-糖基化作用不太明显。这些数据突出了Man 5 GlcNAc 2链坠的不同行为,并提供了蛋白质糖基化功能后果的结构基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号