当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Role of Surface Adsorbed Cl– Complexes in Rechargeable Magnesium Batteries
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-16 , DOI: 10.1021/acscatal.0c01956 Ran Attias 1 , Munseok S. Chae 1 , Ben Dlugatch 1 , Matan Oliel 1 , Yosef Goffer 1 , Doron Aurbach 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-16 , DOI: 10.1021/acscatal.0c01956 Ran Attias 1 , Munseok S. Chae 1 , Ben Dlugatch 1 , Matan Oliel 1 , Yosef Goffer 1 , Doron Aurbach 1
Affiliation
|
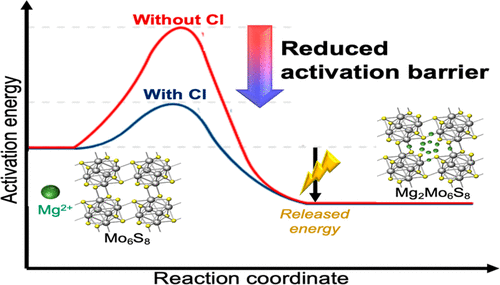
Most electrolyte solutions supporting highly reversible Mg deposition/dissolution contain chlorides. These come as charged or neutral complex species, frequently as Mg-based complex ions, and Lewis-base anions. The electroactive species are usually comprised also of solvent molecules and chlorides as ligands. Numerous studies had shown the critical role of Cl-based species on the electrochemical reaction kinetics, deposition and dissolution overpotentials, electrocrystallization morphology, and many other imperative aspects of the electrochemical responses. Only very few, quite exotic and not fully studied, Cl-free electrolyte solutions have been reported to maintain reversible Mg deposition. Over the years, several compelling theories have been proposed to explain the decisive role of the Cl-containing species on the electrochemical reaction mechanisms during magnesium deposition and dissolution. The role of chlorine-based species in intercalation processes of Mg ions into crystalline hosts was seldom pursued, though. Interestingly, it appears that the Chevrel phase (CP) MgxMo6S8, the main benchmarking Mg intercalation compound, does not intercalate Mg in Cl-free solutions at room temperature (RT). This was established over and again during past decades and recently had been corroborated over a large set of experiments in our lab. Herein, we reveal, via experimental work, that Cl-based species also have a critical role in the electrochemical processes of intercalation. In this work, we studied the function of these during the course of the intercalation process of Mg ions into CP electrodes. We demonstrate that the intercalation process is extremely sluggish, to the point of nonexistent, from Cl-free electrolyte solutions. Quite remarkably, the addition of chlorine-comprising species opens the door to facile magnesium intercalation. On the basis of the numerous experimental results, we suggest a detailed Mg intercalation mechanism into CP. The proposed mechanism emphasizes the importance of the Cl-based species on the charge transfer process across the solid/solution interface. We propose that surface absorbed Cl-containing complexes reduce the activation energy for the interfacial charge transfer stage involving the transport of Mg ions from the solution to the crystalline phase and vice versa. We also propose a relatively simple manner to alleviate the intercalation impediment of Cl-free electrolyte solutions by the cathode’s chemical or electrochemical pretreatment. This project has both scientific and applicative significance and may open a hatch for the future development of practical rechargeable Mg batteries (RMBs).
中文翻译:

表面上吸附氯的作用-场馆在镁二次电池
支持高度可逆的Mg沉积/溶解的大多数电解质溶液都包含氯化物。这些以带电荷或中性的复合物形式出现,通常为基于Mg的复合离子和基于Lewis的阴离子。电活性物质通常还包括溶剂分子和作为配体的氯化物。大量研究表明,基于Cl的物质在电化学反应动力学,沉积和溶解超电势,电结晶形态以及电化学反应的许多其他必要方面具有关键作用。据报道,只有极少数,非常奇特的,尚未充分研究的不含氯的电解质溶液可保持可逆的Mg沉积。这些年来,已经提出了几种令人信服的理论来解释含镁物质在镁沉积和溶解过程中对电化学反应机理的决定性作用。但是,很少追求氯基物质在将Mg离子嵌入晶体主体中的过程中的作用。有趣的是,看起来Chevrel相(CP)MgX沫6小号8,主要的基准Mg嵌入化合物,在室温(RT)下不会嵌入无氯溶液中的Mg。在过去的几十年中,这一点一遍又一遍地建立起来,最近在我们实验室的大量实验中得到了证实。在这里,我们通过实验工作揭示,基于Cl的物质在嵌入的电化学过程中也具有关键作用。在这项工作中,我们研究了这些功能在将Mg离子嵌入CP电极的过程中的功能。我们证明,无氯电解质溶液的插入过程非常缓慢,甚至不存在。十分明显的是,添加了含氯物质为容易插入镁打开了大门。根据大量的实验结果,我们建议将详细的Mg嵌入机制插入CP。拟议中的机制强调了基于Cl的物质在跨固/液界面的电荷转移过程中的重要性。我们提出,表面吸收的含Cl配合物会降低界面电荷转移阶段的活化能,其中涉及Mg离子从溶液到结晶相的转移,反之亦然。我们还提出了一种相对简单的方式来减轻阴极的化学或电化学预处理对无氯电解质溶液的嵌入障碍。该项目具有科学和应用意义,并可能为实用的可充电镁电池(RMB)的未来发展打开大门。拟议中的机制强调了基于Cl的物质在跨固/液界面的电荷转移过程中的重要性。我们提出,表面吸收的含Cl配合物会降低界面电荷转移阶段的活化能,其中涉及Mg离子从溶液到结晶相的转移,反之亦然。我们还提出了一种相对简单的方式来减轻阴极的化学或电化学预处理对无氯电解质溶液的嵌入障碍。该项目具有科学和应用意义,并可能为实用的可充电镁电池(RMB)的未来发展打开大门。拟议中的机制强调了基于Cl的物质在跨固/液界面的电荷转移过程中的重要性。我们提出,表面吸收的含Cl配合物会降低界面电荷转移阶段的活化能,其中涉及Mg离子从溶液到结晶相的转移,反之亦然。我们还提出了一种相对简单的方式来减轻阴极的化学或电化学预处理对无氯电解质溶液的嵌入障碍。该项目具有科学和应用意义,并可能为实用的可充电镁电池(RMB)的未来发展打开大门。我们提出,表面吸收的含Cl配合物会降低界面电荷转移阶段的活化能,其中涉及Mg离子从溶液到结晶相的转移,反之亦然。我们还提出了一种相对简单的方式来减轻阴极化学或电化学预处理对无氯电解质溶液的嵌入障碍。该项目具有科学和应用意义,并可能为实用的可充电镁电池(RMB)的未来发展打开大门。我们提出,表面吸收的含Cl配合物会降低界面电荷转移阶段的活化能,其中涉及Mg离子从溶液到结晶相的转移,反之亦然。我们还提出了一种相对简单的方式来减轻阴极的化学或电化学预处理对无氯电解质溶液的嵌入障碍。该项目具有科学和应用意义,并可能为实用的可充电镁电池(RMB)的未来发展打开大门。
更新日期:2020-07-17
中文翻译:

表面上吸附氯的作用-场馆在镁二次电池
支持高度可逆的Mg沉积/溶解的大多数电解质溶液都包含氯化物。这些以带电荷或中性的复合物形式出现,通常为基于Mg的复合离子和基于Lewis的阴离子。电活性物质通常还包括溶剂分子和作为配体的氯化物。大量研究表明,基于Cl的物质在电化学反应动力学,沉积和溶解超电势,电结晶形态以及电化学反应的许多其他必要方面具有关键作用。据报道,只有极少数,非常奇特的,尚未充分研究的不含氯的电解质溶液可保持可逆的Mg沉积。这些年来,已经提出了几种令人信服的理论来解释含镁物质在镁沉积和溶解过程中对电化学反应机理的决定性作用。但是,很少追求氯基物质在将Mg离子嵌入晶体主体中的过程中的作用。有趣的是,看起来Chevrel相(CP)MgX沫6小号8,主要的基准Mg嵌入化合物,在室温(RT)下不会嵌入无氯溶液中的Mg。在过去的几十年中,这一点一遍又一遍地建立起来,最近在我们实验室的大量实验中得到了证实。在这里,我们通过实验工作揭示,基于Cl的物质在嵌入的电化学过程中也具有关键作用。在这项工作中,我们研究了这些功能在将Mg离子嵌入CP电极的过程中的功能。我们证明,无氯电解质溶液的插入过程非常缓慢,甚至不存在。十分明显的是,添加了含氯物质为容易插入镁打开了大门。根据大量的实验结果,我们建议将详细的Mg嵌入机制插入CP。拟议中的机制强调了基于Cl的物质在跨固/液界面的电荷转移过程中的重要性。我们提出,表面吸收的含Cl配合物会降低界面电荷转移阶段的活化能,其中涉及Mg离子从溶液到结晶相的转移,反之亦然。我们还提出了一种相对简单的方式来减轻阴极的化学或电化学预处理对无氯电解质溶液的嵌入障碍。该项目具有科学和应用意义,并可能为实用的可充电镁电池(RMB)的未来发展打开大门。拟议中的机制强调了基于Cl的物质在跨固/液界面的电荷转移过程中的重要性。我们提出,表面吸收的含Cl配合物会降低界面电荷转移阶段的活化能,其中涉及Mg离子从溶液到结晶相的转移,反之亦然。我们还提出了一种相对简单的方式来减轻阴极的化学或电化学预处理对无氯电解质溶液的嵌入障碍。该项目具有科学和应用意义,并可能为实用的可充电镁电池(RMB)的未来发展打开大门。拟议中的机制强调了基于Cl的物质在跨固/液界面的电荷转移过程中的重要性。我们提出,表面吸收的含Cl配合物会降低界面电荷转移阶段的活化能,其中涉及Mg离子从溶液到结晶相的转移,反之亦然。我们还提出了一种相对简单的方式来减轻阴极的化学或电化学预处理对无氯电解质溶液的嵌入障碍。该项目具有科学和应用意义,并可能为实用的可充电镁电池(RMB)的未来发展打开大门。我们提出,表面吸收的含Cl配合物会降低界面电荷转移阶段的活化能,其中涉及Mg离子从溶液到结晶相的转移,反之亦然。我们还提出了一种相对简单的方式来减轻阴极化学或电化学预处理对无氯电解质溶液的嵌入障碍。该项目具有科学和应用意义,并可能为实用的可充电镁电池(RMB)的未来发展打开大门。我们提出,表面吸收的含Cl配合物会降低界面电荷转移阶段的活化能,其中涉及Mg离子从溶液到结晶相的转移,反之亦然。我们还提出了一种相对简单的方式来减轻阴极的化学或电化学预处理对无氯电解质溶液的嵌入障碍。该项目具有科学和应用意义,并可能为实用的可充电镁电池(RMB)的未来发展打开大门。











































 京公网安备 11010802027423号
京公网安备 11010802027423号