当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Calcium homeostasis plays important roles in the internalization and activities of the small synthetic antifungal peptide PAF26.
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-06-17 , DOI: 10.1111/mmi.14532 Akira J T Alexander 1 , Alberto Muñoz 2 , Jose F Marcos 3 , Nick D Read 4
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-06-17 , DOI: 10.1111/mmi.14532 Akira J T Alexander 1 , Alberto Muñoz 2 , Jose F Marcos 3 , Nick D Read 4
Affiliation
|
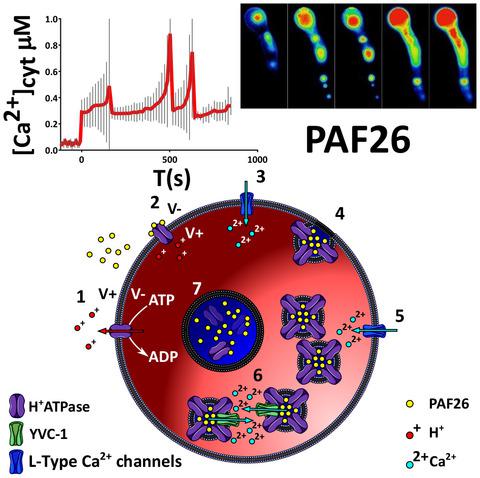
Fungal diseases are responsible for the deaths of over 1.5 million people worldwide annually. Antifungal peptides represent a useful source of antifungals with novel mechanisms‐of‐action, and potentially provide new methods of overcoming resistance. Here we investigate the mode‐of‐action of the small, rationally designed synthetic antifungal peptide PAF26 using the model fungus Neurospora crassa. Here we show that the cell killing activity of PAF26 is dependent on extracellular Ca2+ and the presence of fully functioning fungal Ca2+ homeostatic/signaling machinery. In a screen of mutants with deletions in Ca2+‐signaling machinery, we identified three mutants more tolerant to PAF26. The Ca2+ ATPase NCA‐2 was found to be involved in the initial interaction of PAF26 with the cell envelope. The vacuolar Ca2+ channel YVC‐1 was shown to be essential for its accumulation and concentration within the vacuolar system. The Ca2+ channel CCH‐1 was found to be required to prevent the translocation of PAF26 across the plasma membrane. In the wild type, Ca2+ removal from the medium resulted in the peptide remaining trapped in small vesicles as in the Δyvc‐1 mutant. It is, therefore, apparent that cell killing by PAF26 is complex and unusually dependent on extracellular Ca2+ and components of the Ca2+‐regulatory machinery.
中文翻译:

钙稳态在小型合成抗真菌肽PAF26的内在化和活性中起着重要作用。
每年,真菌疾病导致全球150万人死亡。抗真菌肽代表具有新型作用机制的有用抗真菌来源,并可能提供克服耐药性的新方法。在这里,我们使用真菌真菌Neurospora crassa模型研究了合理设计的小型合成抗真菌肽PAF26的作用方式。在这里,我们显示PAF26的细胞杀伤活性取决于细胞外Ca 2+和功能齐全的真菌Ca 2+稳态/信号传导机制的存在。在筛选具有Ca 2+信号传导机制缺失的突变体中,我们鉴定了三个对PAF26更具耐受性的突变体。Ca 2+发现ATPase NCA-2参与了PAF26与细胞包膜的初始相互作用。液泡Ca 2+通道YVC-1被证明是其在液泡系统中积累和集中所必需的。发现需要Ca 2+通道CCH-1来防止PAF26跨质膜转运。在野生型中,从培养基中去除Ca 2+导致肽保留在小囊泡中,就像Δyvc-1突变体一样。因此,很明显,PAF26杀死细胞非常复杂,并且异常依赖于细胞外Ca 2+和Ca 2+调控机制的成分。
更新日期:2020-06-17
中文翻译:

钙稳态在小型合成抗真菌肽PAF26的内在化和活性中起着重要作用。
每年,真菌疾病导致全球150万人死亡。抗真菌肽代表具有新型作用机制的有用抗真菌来源,并可能提供克服耐药性的新方法。在这里,我们使用真菌真菌Neurospora crassa模型研究了合理设计的小型合成抗真菌肽PAF26的作用方式。在这里,我们显示PAF26的细胞杀伤活性取决于细胞外Ca 2+和功能齐全的真菌Ca 2+稳态/信号传导机制的存在。在筛选具有Ca 2+信号传导机制缺失的突变体中,我们鉴定了三个对PAF26更具耐受性的突变体。Ca 2+发现ATPase NCA-2参与了PAF26与细胞包膜的初始相互作用。液泡Ca 2+通道YVC-1被证明是其在液泡系统中积累和集中所必需的。发现需要Ca 2+通道CCH-1来防止PAF26跨质膜转运。在野生型中,从培养基中去除Ca 2+导致肽保留在小囊泡中,就像Δyvc-1突变体一样。因此,很明显,PAF26杀死细胞非常复杂,并且异常依赖于细胞外Ca 2+和Ca 2+调控机制的成分。











































 京公网安备 11010802027423号
京公网安备 11010802027423号