当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Density functional theory studies of effects of boron replacement on the structure and property of RDX and HMX
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-06-16 , DOI: 10.1002/jccs.201900399 Qiong Wu 1 , Gaojie Yan 1 , Mingquan Li 1 , Qinnan Hu 1 , Zewu Zhang 1 , Weihua Zhu 2
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-06-16 , DOI: 10.1002/jccs.201900399 Qiong Wu 1 , Gaojie Yan 1 , Mingquan Li 1 , Qinnan Hu 1 , Zewu Zhang 1 , Weihua Zhu 2
Affiliation
|
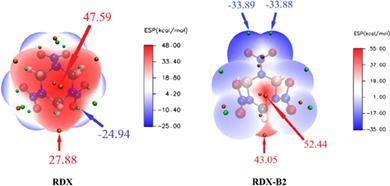
In this study, based on two model nitramine compounds hexahydro‐1,3,5‐trinitro‐1,3,5‐triazine (RDX) and octahydro‐1,3,5,7‐tetranitro‐1,3,5, 7‐tetrazocine (HMX), two series of new energetic molecules were designed by replacing carbon atoms in the ring with different amounts of boron atoms, their structures and performances were investigated theoretically by the density functional theory method. The results showed that the boron replacement could affect the molecular shape and electronic structure of RDX and HMX greatly, and then would do harm to the main performance like the heat of formation, density, and sensitivity. However, the compound RDX‐B2 is an exception; it was formed by replacing two boron atoms into the system of RDX and has the symmetric boat‐like structure. Its oxygen balance (4.9%), density (1.91 g/cm3), detonation velocity (8.85 km/s), and detonation pressure (36.9 GPa) are all higher than RDX. Furthermore, RDX‐B2 has shorter and stronger NNO2 bonds than RDX, making it possesses lower sensitivity (45 cm) and better thermal stability (the bond dissociation energy for the NNO2 bond is 204.7 kJ/mol) than RDX. Besides, RDX‐B1 and HMX‐B4 also have good overall performance; these three new molecules may be regarded as a new potential candidate for high energy density compounds.
中文翻译:

硼置换对RDX和HMX结构和性能影响的密度泛函理论研究
在本研究中,基于两种模型的硝胺化合物六氢-1,3,5-三硝基-1,3,5-三嗪(RDX)和八氢-1,3,5,7-四硝基-1,3,5,7通过使用不同量的硼原子取代环中的碳原子,设计了两个系列的四氮唑啉(HMX)高能分子,并通过密度泛函理论方法对其结构和性能进行了理论研究。结果表明,硼的替代会极大地影响RDX和HMX的分子形状和电子结构,进而损害其主要性能,如形成热,密度和敏感性。但是,复合RDX-B2是一个例外。它是通过将两个硼原子置换为RDX体系而形成的,具有对称的船形结构。其氧平衡(4.9%),密度(1.91 g / cm 3),爆炸速度(8.85 km / s)和爆炸压力(36.9 GPa)均高于RDX。此外,RDX-B2具有较短的和更强的Ñ NO 2个比RDX键,使得它具有较低的敏感性(45厘米),更好的热稳定性(在键离解能为所述N个 NO 2键204.7千焦/摩尔)比RDX 。此外,RDX‐B1和HMX‐B4也具有良好的整体性能。这三个新分子可被视为高能量密度化合物的新潜在候选者。
更新日期:2020-06-16
中文翻译:

硼置换对RDX和HMX结构和性能影响的密度泛函理论研究
在本研究中,基于两种模型的硝胺化合物六氢-1,3,5-三硝基-1,3,5-三嗪(RDX)和八氢-1,3,5,7-四硝基-1,3,5,7通过使用不同量的硼原子取代环中的碳原子,设计了两个系列的四氮唑啉(HMX)高能分子,并通过密度泛函理论方法对其结构和性能进行了理论研究。结果表明,硼的替代会极大地影响RDX和HMX的分子形状和电子结构,进而损害其主要性能,如形成热,密度和敏感性。但是,复合RDX-B2是一个例外。它是通过将两个硼原子置换为RDX体系而形成的,具有对称的船形结构。其氧平衡(4.9%),密度(1.91 g / cm 3),爆炸速度(8.85 km / s)和爆炸压力(36.9 GPa)均高于RDX。此外,RDX-B2具有较短的和更强的Ñ NO 2个比RDX键,使得它具有较低的敏感性(45厘米),更好的热稳定性(在键离解能为所述N个 NO 2键204.7千焦/摩尔)比RDX 。此外,RDX‐B1和HMX‐B4也具有良好的整体性能。这三个新分子可被视为高能量密度化合物的新潜在候选者。



























 京公网安备 11010802027423号
京公网安备 11010802027423号