当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functionalized Chiral Bambusurils: Synthesis and Host-Guest Interactions with Chiral Carboxylates.
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-06-17 , DOI: 10.1002/cplu.202000261 Jan Sokolov 1 , Adam Štefek 1 , Vladimír Šindelář 1
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-06-17 , DOI: 10.1002/cplu.202000261 Jan Sokolov 1 , Adam Štefek 1 , Vladimír Šindelář 1
Affiliation
|
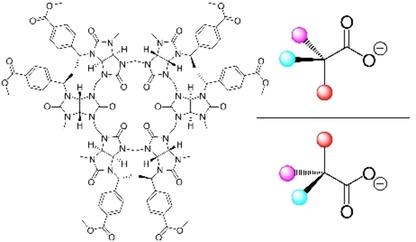
Bambusurils are a class of macrocyclic anion receptors that exhibit notable anion recognition properties, able to bind various inorganic anions as well the carboxylates or sulfonates. Recently, we reported enantioselective recognition of chiral carboxylates using non‐functionalized chiral bambusuril derivatives. Herein, we report the synthesis and host‐guest properties of two new representatives of chiral bambusuril macrocycles bearing ester functional groups, differing by the substituents attached to their portals. Their supramolecular properties in terms of carboxylate binding were studied by means of NMR in DMSO‐d 6. The reported bambusurils bind selected chiral carboxylates with enantioselectivity factors up to 3.1. The results indicated that the selectivity towards different carboxylates is governed by the steric constraint of the substituents surrounding bambusuril portals. No clear trend in the binding affinities and their enantioselectivities was found.
中文翻译:

功能化的手性小铃虫:手性羧酸的合成和宿主与客体的相互作用。
氨苄青霉素是一类具有显着阴离子识别特性的大环阴离子受体,能够与各种无机阴离子以及羧酸盐或磺酸盐结合。最近,我们报道了使用非功能化手性bambusuril衍生物对手性羧酸酯的对映选择性识别。本文中,我们报道了两个新的具有酯官能团的手性bambusuril大环代表化合物的合成和客体特性,它们之间的取代基不同。通过DMSO- d 6中的NMR研究了它们在羧酸盐结合方面的超分子特性。报道的樟脑丸以高达3.1的对映选择性因子结合选择的手性羧酸盐。结果表明,对不同羧酸盐的选择性受孟买素门户周围的取代基的空间约束所支配。没有发现结合亲和力及其对映选择性的明显趋势。
更新日期:2020-06-17
中文翻译:

功能化的手性小铃虫:手性羧酸的合成和宿主与客体的相互作用。
氨苄青霉素是一类具有显着阴离子识别特性的大环阴离子受体,能够与各种无机阴离子以及羧酸盐或磺酸盐结合。最近,我们报道了使用非功能化手性bambusuril衍生物对手性羧酸酯的对映选择性识别。本文中,我们报道了两个新的具有酯官能团的手性bambusuril大环代表化合物的合成和客体特性,它们之间的取代基不同。通过DMSO- d 6中的NMR研究了它们在羧酸盐结合方面的超分子特性。报道的樟脑丸以高达3.1的对映选择性因子结合选择的手性羧酸盐。结果表明,对不同羧酸盐的选择性受孟买素门户周围的取代基的空间约束所支配。没有发现结合亲和力及其对映选择性的明显趋势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号