当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed C-H Iodination of Arenes by Means of Sulfinyl Directing Groups.
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-06-17 , DOI: 10.1002/asia.202000591 Hayate Saito,Keita Yamamoto,Yosuke Sumiya,Ling-Jun Liu,Keisuke Nogi,Satoshi Maeda,Hideki Yorimitsu
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-06-17 , DOI: 10.1002/asia.202000591 Hayate Saito,Keita Yamamoto,Yosuke Sumiya,Ling-Jun Liu,Keisuke Nogi,Satoshi Maeda,Hideki Yorimitsu
|
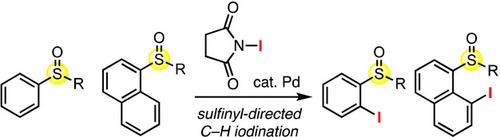
C−H iodination of aromatic compounds has been accomplished with the aid of sulfinyl directing groups under palladium catalysis. The reaction proceeds selectively at the peri‐position of polycyclic aryl sulfoxides or at the ortho‐position of phenyl sulfoxides. The iodination products can be further converted via iterative catalytic cross‐coupling at the expense of the C−I and C−S bonds. Computational studies suggest that peri‐C−H palladation would proceed via a non‐directed pathway, wherein neither of the sulfur nor oxygen atom of the sulfinyl group coordinates to the palladium before and at the transition state.
中文翻译:

借助于亚磺酰基导向基团,钯催化芳烃的CH碘化。
芳族化合物的CHH碘化反应是在钯催化下借助亚磺酰基导向基团完成的。该反应在多环芳基亚砜的周边或苯基亚砜的邻位选择性进行。碘化产物可以通过迭代催化交叉偶联进一步转化,但以CI和CS键为代价。计算研究表明,围-C-H palladation将着手通过非定向的通路,其特征在于,既不之前和在过渡状态下的亚磺酰基坐标到钯的硫也不氧原子。
更新日期:2020-08-18
中文翻译:

借助于亚磺酰基导向基团,钯催化芳烃的CH碘化。
芳族化合物的CHH碘化反应是在钯催化下借助亚磺酰基导向基团完成的。该反应在多环芳基亚砜的周边或苯基亚砜的邻位选择性进行。碘化产物可以通过迭代催化交叉偶联进一步转化,但以CI和CS键为代价。计算研究表明,围-C-H palladation将着手通过非定向的通路,其特征在于,既不之前和在过渡状态下的亚磺酰基坐标到钯的硫也不氧原子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号