当前位置:
X-MOL 学术
›
Biotechnol. Appl. Bioc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Promoting P450 BM3 heme domain dimerization with a tris(5‐iodoacetamido‐1,10‐phenanthroline)Ru(II) complex
Biotechnology and Applied Biochemistry ( IF 3.2 ) Pub Date : 2020-06-17 , DOI: 10.1002/bab.1970 Mallory Kato 1 , Bridget Foley 1 , Julia Vu 1 , Michael Huynh 1 , Kathreena Lucero 1 , Caroline Harmon 1 , Lionel Cheruzel 1
Biotechnology and Applied Biochemistry ( IF 3.2 ) Pub Date : 2020-06-17 , DOI: 10.1002/bab.1970 Mallory Kato 1 , Bridget Foley 1 , Julia Vu 1 , Michael Huynh 1 , Kathreena Lucero 1 , Caroline Harmon 1 , Lionel Cheruzel 1
Affiliation
|
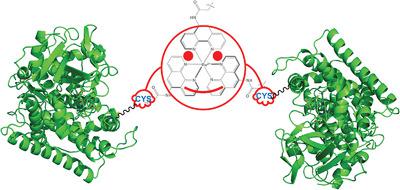
Protein dimerization often occurs in many biological systems as to provide structural and functional advantages. A tris(5‐iodoacetamido‐1,10‐phenanthroline)Ruthenium(II) complex was shown to promote the covalent dimerization of a P450 BM3 heme domain mutant containing a surface‐exposed nonnative single cysteine residue. The formation of homodimeric species was confirmed by protein gel electrophoresis, mass spectrometry and UV–Vis spectroscopy. The dimeric species could be separated from the monomer and aggregates by size‐exclusion chromatography. Docking simulation reveals a plausible structure with two proteins covalently conjugated to the inorganic compound.
中文翻译:

用三(5-碘乙酰氨基-1,10-菲咯啉)Ru(II)配合物促进P450 BM3血红素域二聚化
蛋白质二聚化经常发生在许多生物系统中,以提供结构和功能上的优势。三(5-碘乙酰氨基-1,10-菲咯啉)钌(II)配合物可促进含有表面暴露的非天然单半胱氨酸残基的P450 BM3血红素结构域突变体的共价二聚化。同源二聚体物种的形成已通过蛋白凝胶电泳,质谱和UV-Vis光谱确认。可以通过尺寸排阻色谱法将二聚体与单体和聚集体分离。对接模拟显示了一种合理的结构,其中两种蛋白质共价结合到无机化合物上。
更新日期:2020-06-17
中文翻译:

用三(5-碘乙酰氨基-1,10-菲咯啉)Ru(II)配合物促进P450 BM3血红素域二聚化
蛋白质二聚化经常发生在许多生物系统中,以提供结构和功能上的优势。三(5-碘乙酰氨基-1,10-菲咯啉)钌(II)配合物可促进含有表面暴露的非天然单半胱氨酸残基的P450 BM3血红素结构域突变体的共价二聚化。同源二聚体物种的形成已通过蛋白凝胶电泳,质谱和UV-Vis光谱确认。可以通过尺寸排阻色谱法将二聚体与单体和聚集体分离。对接模拟显示了一种合理的结构,其中两种蛋白质共价结合到无机化合物上。









































 京公网安备 11010802027423号
京公网安备 11010802027423号