当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antagomir-155 Attenuates Acute Cardiac Rejection Using Ultrasound Targeted Microbubbles Destruction.
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2020-06-16 , DOI: 10.1002/adhm.202000189 Luyang Yi 1, 2 , Yihan Chen 1, 2 , Qiaofeng Jin 1, 2 , Cheng Deng 1, 2 , Ya Wu 1, 2 , Huiling Li 1, 2 , Tianshu Liu 1, 2 , Yuman Li 1, 2 , Yali Yang 1, 2 , Jing Wang 1, 2 , Qing Lv 1, 2 , Li Zhang 1, 2 , Mingxing Xie 1, 2
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2020-06-16 , DOI: 10.1002/adhm.202000189 Luyang Yi 1, 2 , Yihan Chen 1, 2 , Qiaofeng Jin 1, 2 , Cheng Deng 1, 2 , Ya Wu 1, 2 , Huiling Li 1, 2 , Tianshu Liu 1, 2 , Yuman Li 1, 2 , Yali Yang 1, 2 , Jing Wang 1, 2 , Qing Lv 1, 2 , Li Zhang 1, 2 , Mingxing Xie 1, 2
Affiliation
|
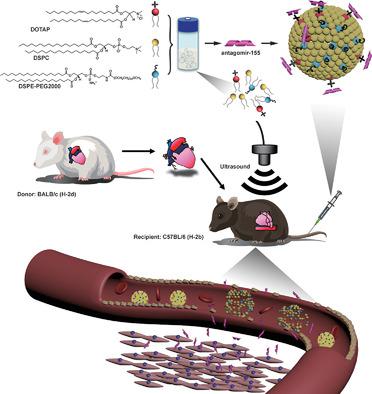
Antagomir‐155 is an artificial inhibitor of miRNA‐155, which is expected to be a promising therapeutic target to attenuate acute cardiac rejection (ACR). However, its vulnerability of being degraded by endogenous nuclease and potential off‐target effect make the authors seek for a more suitable way to delivery it. In attribution of efficiency and safety, ultrasound targeted microbubbles destruction (UTMD) turns out to be an appropriate method to deliver gene to target tissues. Here, cationic microbubbles to deliver antagomir‐155 downregulating miRNA‐155 in murine allograft hearts triggered by UTMD are synthesized. The viability of this therapy is verified by fluorescent microscopy. The biodistribution of antagomir‐155 is analyzed by optical imaging system. The results show antagomir‐155 delivered by UTMD which significantly decreases the levels of miR‐155. Also, this therapy downregulates the expression of cytokines and inflammation infiltration. And allograft survival time is significantly prolonged. Therefore, antagomir‐loaded microbubbles trigged by UTMD may provide a novel platform for ACR target treatment.
中文翻译:

Antagomir-155使用超声靶向微泡破坏来减轻急性心脏排斥反应。
Antagomir-155是miRNA-155的人工抑制剂,有望成为减轻急性心脏排斥反应(ACR)的有希望的治疗靶标。然而,其易被内源性核酸酶降解的脆弱性和潜在的脱靶效应使作者寻求一种更合适的方式来递送它。为了提高效率和安全性,超声靶向微泡破坏(UTMD)被证明是将基因传递至靶组织的合适方法。在这里,合成了阳离子微泡以在UTMD触发的小鼠同种异体心脏中递送antagomir-155下调miRNA-155。该疗法的可行性通过荧光显微镜证实。通过光学成像系统分析了antagomir-155的生物分布。结果表明,UTMD递送了antagomir-155,这大大降低了miR-155的水平。同样,该疗法下调细胞因子的表达和炎症浸润。并且同种异体移植的存活时间显着延长。因此,由UTMD触发的载有antagomir的微泡可能为ACR靶标治疗提供了一个新颖的平台。
更新日期:2020-07-22
中文翻译:

Antagomir-155使用超声靶向微泡破坏来减轻急性心脏排斥反应。
Antagomir-155是miRNA-155的人工抑制剂,有望成为减轻急性心脏排斥反应(ACR)的有希望的治疗靶标。然而,其易被内源性核酸酶降解的脆弱性和潜在的脱靶效应使作者寻求一种更合适的方式来递送它。为了提高效率和安全性,超声靶向微泡破坏(UTMD)被证明是将基因传递至靶组织的合适方法。在这里,合成了阳离子微泡以在UTMD触发的小鼠同种异体心脏中递送antagomir-155下调miRNA-155。该疗法的可行性通过荧光显微镜证实。通过光学成像系统分析了antagomir-155的生物分布。结果表明,UTMD递送了antagomir-155,这大大降低了miR-155的水平。同样,该疗法下调细胞因子的表达和炎症浸润。并且同种异体移植的存活时间显着延长。因此,由UTMD触发的载有antagomir的微泡可能为ACR靶标治疗提供了一个新颖的平台。









































 京公网安备 11010802027423号
京公网安备 11010802027423号