Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-06-17 , DOI: 10.1016/j.tetlet.2020.152152 Yuki Murata , Aki Terazoe , Misato Kiba , Yuki Kitamura , Mio Matsumura , Shuji Yasuike
|
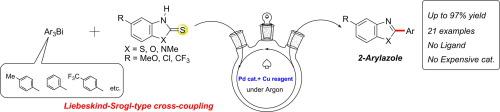
Liebeskind-Srogl-type C(HetAr)–C(Ar) bond formation using trivalent organobismuth compounds as a new class of arylating reagents is described. The reaction of benzazole-2-thiones with triarylbismuthines in the presence of 10 mol% Pd(dba)2 and 2.0 equiv. Cu(OAc)2 at 80 °C affords 2-arylbenzothiazoles, benzoxazoles, and N-methyl benzimidazole in moderate-to-high yield. The reaction is sensitive to the electronic nature of triarylbismuthines: compounds bearing an electron-withdrawing group on the phenyl ring showed higher reactivity than those having an electron-donating group.
中文翻译:

唑-2-硫酮与三芳基双变异蛋白的Liebeskind-Srogl型交叉偶联反应:2-芳基唑的合成
描述了使用三价有机铋化合物作为新型芳构化试剂的Liebeskind-Srogl型C(HetAr)–C(Ar)键形成。在10 mol%Pd(dba)2和2.0当量的存在下,苯并恶唑-2-硫酮与三芳基双突变体的反应。Cu(OAc)2在80°C时以中到高收率提供2-芳基苯并噻唑,苯并恶唑和N-甲基苯并咪唑。该反应对三芳基双突变体的电子性质敏感:在苯环上带有吸电子基团的化合物显示出比具有供电子基团的化合物更高的反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号