Industrial Crops and Products ( IF 5.9 ) Pub Date : 2020-06-16 , DOI: 10.1016/j.indcrop.2020.112620 Shuvobrata Majumder , Chirabrata Sarkar , Karabi Datta , Swapan K. Datta
|
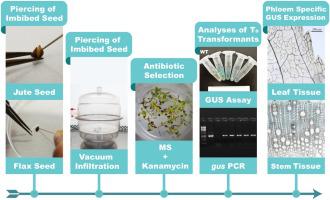
Jute (Corchorus capsularis) and flax (Linum usitatissimum) have been established as important sources of bast fibres (phloem fibres) worldwide. These long and lustrous natural fibres are used for various commercial and industrial purposes and hold immense future prospects. To meet the worldwide demand for bast fibres, improvement in the production and quality of jute and flax is essential. Molecular breeding is an expeditious approach for varietal improvement and requires an efficient gene transformation system. The available methods for jute and flax transformation are highly specific to species and variety, requiring a long time and rigorous preparation of media, explants, and screening procedures. In this study, we used a fast and stable method for Agrobacterium tumefaciens-mediated transformation of jute (variety JRC321) and flax (variety FT-897), known as the ‘imbibed seed piercing’ method (ISPM), where seeds were used as explants. A phloem-specific Arabidopsis sucrose-proton H+ symporter 2 (AtSUC2) promoter-driven gus gene (β-glucuronidase) was used for the assessment of genetic transformation of jute and flax plants. Transformed seedlings were selected following three consecutive selection pressure tests conducted at two-week intervals using kanamycin-supplemented culture media. Transgene integration was verified using Southern blotting and PCR-based molecular characterization of jute and flax plants. The gus gene expression levels in the transgenic jute and flax tissue samples were analysed by semi-quantitative and quantitative RT-PCR. The GUS protein expression in transgenic plant tissues was confirmed by the development of blue colour in the histochemical GUS assay. Blue colouration was observed only in phloem cells of the tested plant tissues. Transgenic progeny plants of subsequent generations followed the 3:1 transgene segregation pattern and exhibited phloem-specific GUS expression. This fast, easy, and stable ISPM could also be applied to other fibre producing industrial crops and could form a part of a modern CRISPR/Cas9-based genome editing tool by efficiently delivering CRISPR vectors in jute and flax plants.
中文翻译:

通过韧皮部特异性表达β-葡萄糖醛酸苷酶基因建立农杆菌介导的黄麻和韧皮纤维农作物“吸水种子穿刺”方法的建立
黄麻(Corchorus cystris)和亚麻(Linum usitatissimum)已被确定为全世界韧皮纤维(韧皮部纤维)的重要来源。这些长而有光泽的天然纤维可用于各种商业和工业用途,并具有广阔的前景。为了满足全世界对韧皮纤维的需求,提高黄麻和亚麻的产量和质量至关重要。分子育种是用于品种改良的快速方法,并且需要有效的基因转化系统。黄麻和亚麻转化的可用方法对物种和品种具有高度特异性,需要长时间和严格准备培养基,外植体和筛选程序。在这项研究中,我们使用了一种快速稳定的方法农杆菌介导的黄麻(品种JRC321)和亚麻(品种FT-897)介导的转化,称为“浸种种子刺穿”方法(ISPM),其中种子用作外植体。韧皮部特异性拟南芥蔗糖质子H +转运蛋白2(AtSUC2)启动子驱动的gus基因(β-葡萄糖醛酸苷酶)用于评估黄麻和亚麻植物的遗传转化。在使用卡那霉素补充的培养基中,每两周间隔进行三个连续的选择压力试验,选择转化的幼苗。使用Southern印迹和基于黄麻和亚麻植物的PCR分子表征验证了转基因整合。该GUS通过半定量和定量RT-PCR分析转基因黄麻和亚麻组织样品中的基因表达水平。在组织化学GUS分析中,蓝色的发展证实了转基因植物组织中GUS蛋白的表达。仅在被测植物组织的韧皮部细胞中观察到蓝色。后代的转基因后代植物遵循3:1转基因分离模式,并显示韧皮部特异性GUS表达。这种快速,简便和稳定的ISPM也可以应用于其他生产纤维的工业作物,并且可以通过在黄麻和亚麻植物中高效递送CRISPR载体,构成基于CRISPR / Cas9的现代基因组编辑工具的一部分。



























 京公网安备 11010802027423号
京公网安备 11010802027423号