当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The temperature of maximum density for amino acid aqueous solutions. An experimental and molecular dynamics study
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.fluid.2020.112703 Diego González-Salgado , Jacobo Troncoso , Enrique Lomba
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.fluid.2020.112703 Diego González-Salgado , Jacobo Troncoso , Enrique Lomba
|
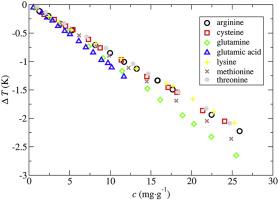
Abstract The temperature of maximum density (TMD) for aqueous solutions of seven amino acids has been experimentally determined by means of density measurements versus temperature. The selected amino acids have been arginine, cysteine, glutamic acid, glutamine, lysine, methionine, and threonine. The TMD dependence against composition has been obtained from the experimental data and characterized through the Despretz constant. All amino acids induce a depression in the TMD as compared with that of pure water. If the mole fraction is selected as composition variable, a clear dependence against amino acid molar mass is observed, which disappears when TMDs are represented versus mass fraction; almost all data shrink onto a single straight line. It must be pointed out that the TMD depressions for all studied amino acids are quite greater than those previously observed for proteins. This suggests that TMDs for proteins cannot be explained as a simple, additive result of its constituents, and, therefore complex cooperative phenomena seem to take place. The partial molar volume at infinite dilution has been obtained from density data, and a consistency test between its temperature dependence and that of temperature of maximum density versus composition has been performed, obtaining satisfactory results. A molecular dynamics study for all the studied systems has been also carried out. Amino acids have been modeled through the OPLS-AA force field, whereas the TIP4P/2005 model was used for water. The temperature of maximum density and partial molar volume have been calculated from the simulated density, and the results are compared with experimental data. Although the agreement is only fair, similar qualitative trends were obtained. The simulated Despretz constants are smaller than the experimental ones, a result that was already previously observed for methanol aqueous solutions. A structural analysis of water molecules in solution along the MD trajectories showed no enhancement of ice-like structures in complete agreement with the TMD decrease with concentration.
中文翻译:

氨基酸水溶液的最大密度温度。实验和分子动力学研究
摘要 七种氨基酸水溶液的最大密度 (TMD) 温度已通过密度测量与温度的关系通过实验确定。选定的氨基酸是精氨酸、半胱氨酸、谷氨酸、谷氨酰胺、赖氨酸、甲硫氨酸和苏氨酸。TMD 对成分的依赖性已从实验数据中获得,并通过 Despretz 常数表征。与纯水相比,所有氨基酸都会导致 TMD 降低。如果选择摩尔分数作为组成变量,则观察到对氨基酸摩尔质量的明显依赖性,当 TMD 与质量分数表示时,这种依赖性消失;几乎所有数据都缩小到一条直线上。必须指出的是,所有研究的氨基酸的 TMD 抑制比以前观察到的蛋白质大得多。这表明蛋白质的 TMD 不能解释为其成分的简单、相加结果,因此似乎发生了复杂的合作现象。从密度数据得到无限稀释时的偏摩尔体积,并对其温度依赖性与最大密度温度与组成的温度依赖性进行了一致性测试,获得了满意的结果。还对所有研究的系统进行了分子动力学研究。氨基酸已通过 OPLS-AA 力场建模,而 TIP4P/2005 模型用于水。根据模拟密度计算了最大密度和偏摩尔体积的温度,并将结果与实验数据进行了比较。尽管该协议只是公平的,但获得了类似的定性趋势。模拟的 Despretz 常数比实验的要小,之前已经在甲醇水溶液中观察到了这一结果。沿着 MD 轨迹对溶液中水分子的结构分析表明,冰状结构没有增强,这与 TMD 随浓度的降低完全一致。先前已经在甲醇水溶液中观察到的结果。沿着 MD 轨迹对溶液中水分子的结构分析表明,冰状结构没有增强,这与 TMD 随浓度的降低完全一致。先前已在甲醇水溶液中观察到的结果。沿着 MD 轨迹对溶液中水分子的结构分析表明,冰状结构没有增强,这与 TMD 随浓度的降低完全一致。
更新日期:2020-10-01
中文翻译:

氨基酸水溶液的最大密度温度。实验和分子动力学研究
摘要 七种氨基酸水溶液的最大密度 (TMD) 温度已通过密度测量与温度的关系通过实验确定。选定的氨基酸是精氨酸、半胱氨酸、谷氨酸、谷氨酰胺、赖氨酸、甲硫氨酸和苏氨酸。TMD 对成分的依赖性已从实验数据中获得,并通过 Despretz 常数表征。与纯水相比,所有氨基酸都会导致 TMD 降低。如果选择摩尔分数作为组成变量,则观察到对氨基酸摩尔质量的明显依赖性,当 TMD 与质量分数表示时,这种依赖性消失;几乎所有数据都缩小到一条直线上。必须指出的是,所有研究的氨基酸的 TMD 抑制比以前观察到的蛋白质大得多。这表明蛋白质的 TMD 不能解释为其成分的简单、相加结果,因此似乎发生了复杂的合作现象。从密度数据得到无限稀释时的偏摩尔体积,并对其温度依赖性与最大密度温度与组成的温度依赖性进行了一致性测试,获得了满意的结果。还对所有研究的系统进行了分子动力学研究。氨基酸已通过 OPLS-AA 力场建模,而 TIP4P/2005 模型用于水。根据模拟密度计算了最大密度和偏摩尔体积的温度,并将结果与实验数据进行了比较。尽管该协议只是公平的,但获得了类似的定性趋势。模拟的 Despretz 常数比实验的要小,之前已经在甲醇水溶液中观察到了这一结果。沿着 MD 轨迹对溶液中水分子的结构分析表明,冰状结构没有增强,这与 TMD 随浓度的降低完全一致。先前已经在甲醇水溶液中观察到的结果。沿着 MD 轨迹对溶液中水分子的结构分析表明,冰状结构没有增强,这与 TMD 随浓度的降低完全一致。先前已在甲醇水溶液中观察到的结果。沿着 MD 轨迹对溶液中水分子的结构分析表明,冰状结构没有增强,这与 TMD 随浓度的降低完全一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号