Computational and Theoretical Chemistry ( IF 3.0 ) Pub Date : 2020-06-17 , DOI: 10.1016/j.comptc.2020.112911 Mina Ghiasi , Samira Gholami
|
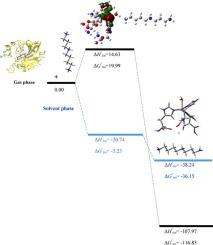
Spermine and spermidine polyamines are investigated as a new class inhibitors of different carbonic anhydrase (CA) isoforms using DFT calculations. Our results indicate these two polyamines, inhibited the human carbonic anhydrase with very different inhibition profiles compared to other inhibitors. According to calculated results, studied polyamines are anchored to the non-protein zinc ligand (hydroxyl ion) by means of a hydrogen bond of 2.6 Å involving one of terminal ammonium group and an intermediate complex is formed, [(his)3Zn(II)(OH)/polyamine], which is in good agreement with experimental data. By transferring a proton from the terminal ammonium group of polyamine inhibitor to the oxygen atom of the hydroxyl group, the active form of the CA enzyme converted to the inactive form. Finally, the HOMO-LUMO and AIM analysis have been done to understand the details of interaction between studied polyamines and CA active center in intermediate complex in water phase.
中文翻译:

人碳酸酐酶Ⅱ与多胺复合作为新型抑制剂的量子力学研究:动力学和热力学研究
使用DFT计算研究了精胺和亚精胺多胺作为不同碳酸酐酶(CA)同工型的新型抑制剂。我们的结果表明,与其他抑制剂相比,这两种多胺抑制人碳酸酐酶的抑制曲线非常不同。根据计算结果,所研究的多胺通过2.6 A的氢键与一个末端铵基团结合而锚定在非蛋白质锌配体(羟基离子)上,并形成中间配合物,[(his)3Zn(II)(OH)/多胺],与实验数据非常吻合。通过将质子从多胺抑制剂的末端铵基转移至羟基的氧原子,CA酶的活性形式转化为无活性形式。最后,进行了HOMO-LUMO和AIM分析,以了解所研究的多胺与CA活性中心在水相中间复合物中的相互作用的细节。









































 京公网安备 11010802027423号
京公网安备 11010802027423号