Chemical Physics Letters ( IF 2.8 ) Pub Date : 2020-06-17 , DOI: 10.1016/j.cplett.2020.137719 Meili Liu , Hongwei Tan , Guangju Chen
|
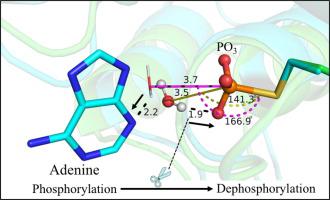
The mechanistic role of adenine, which assists the catalytic activity of low-molecular weight phosphatases, has been investigated using ONIOM calculations. Results confirm the dephosphorylation step being the rate-limiting step. In the absence and presence of adenine, the energy barriers of the rate-limiting step are 16.0 kcal/mol and 14.3 kcal/mol respectively, in agreement with experimental data. The formation of the favorable hydrogen bond contributes to the decreasing of barrier energy, resulting in the optimized attacking angle of the nucleophile. These results support the notion that adenine disfavors the formation of adverse hydrogen bond, which enables more effective hydrolyzation of phosphoenzyme intermediate.
中文翻译:

腺嘌呤促进低分子酪氨酸磷酸酶活性的机制研究:ONIOM研究
腺嘌呤的机制作用,有助于低分子量磷酸酶的催化活性,已使用ONIOM计算进行了研究。结果证实脱磷酸化步骤是限速步骤。在不存在和存在腺嘌呤的情况下,限速步骤的能垒分别为16.0 kcal / mol和14.3 kcal / mol,与实验数据一致。有利氢键的形成有助于降低势垒能量,从而导致亲核试剂的最佳攻角。这些结果支持了腺嘌呤不利于不利的氢键形成的观点,这使得能够更有效地水解磷酸酶中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号