当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sulfur-mediated synthesis of unsymmetrically substituted N-aryl oxalamides by the cascade thioamidation/cyclocondensation and hydrolysis reaction.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-06-16 , DOI: 10.1039/d0ob00811g Tatyana A Tikhonova 1 , Nikita V Ilment 1 , Konstantin A Lyssenko 2 , Igor V Zavarzin 1 , Yulia A Volkova 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-06-16 , DOI: 10.1039/d0ob00811g Tatyana A Tikhonova 1 , Nikita V Ilment 1 , Konstantin A Lyssenko 2 , Igor V Zavarzin 1 , Yulia A Volkova 1
Affiliation
|
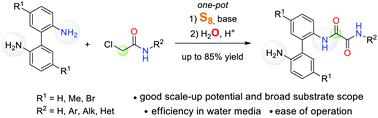
A facile and straightforward synthesis of unsymmetrically substituted N-aryl oxalamides from 2,2′-biphenyldiamines, 2-chloroacetic acid derivatives, elemental sulfur, and water has been developed. This protocol is distinguished by efficiency in water under metal-free conditions for N-aryl oxalamides bearing a side-chain NH2-group; it can be adapted for scale-up synthesis. The scope and limitations of this transformation have been investigated.
中文翻译:

通过级联的硫酰胺化/环缩合和水解反应进行硫介导的不对称取代的N-芳基草酰胺的合成。
已开发了一种由2,2'-联苯二胺,2-氯乙酸衍生物,元素硫和水轻松快速地合成不对称取代的N-芳基草酰胺的方法。该方案的特点是在无金属条件下水中带有侧链NH 2-基团的N-芳基草酰胺的效率。它可以适用于按比例放大的合成。已经研究了这种转变的范围和局限性。
更新日期:2020-07-08
中文翻译:

通过级联的硫酰胺化/环缩合和水解反应进行硫介导的不对称取代的N-芳基草酰胺的合成。
已开发了一种由2,2'-联苯二胺,2-氯乙酸衍生物,元素硫和水轻松快速地合成不对称取代的N-芳基草酰胺的方法。该方案的特点是在无金属条件下水中带有侧链NH 2-基团的N-芳基草酰胺的效率。它可以适用于按比例放大的合成。已经研究了这种转变的范围和局限性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号