当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comparison of the impact of ozone, chlorine dioxide, ferrate and permanganate pre-oxidation on organic disinfection byproduct formation during post-chlorination
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2020-06-16 , DOI: 10.1039/d0ew00411a Valentin Rougé 1, 2, 3, 4 , Urs von Gunten 5, 6, 7, 8, 9 , Mariette Lafont de Sentenac 1, 2, 3, 4 , Massimiliano Massi 1, 2, 3, 4 , Phillip J. Wright 1, 2, 3, 4 , Jean-Philippe Croué 1, 2, 3, 4, 10 , Sébastien Allard 1, 2, 3, 4
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2020-06-16 , DOI: 10.1039/d0ew00411a Valentin Rougé 1, 2, 3, 4 , Urs von Gunten 5, 6, 7, 8, 9 , Mariette Lafont de Sentenac 1, 2, 3, 4 , Massimiliano Massi 1, 2, 3, 4 , Phillip J. Wright 1, 2, 3, 4 , Jean-Philippe Croué 1, 2, 3, 4, 10 , Sébastien Allard 1, 2, 3, 4
Affiliation
|
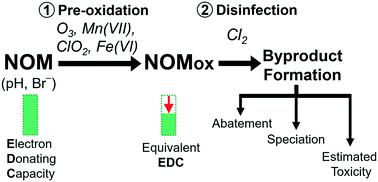
Pre-oxidation is commonly used to mitigate the formation of byproducts during post-disinfection. A comparative study of the impact of four pre-oxidants, ozone (O3), chlorine dioxide (ClO2), permanganate (Mn(VII)) and ferrate (Fe(VI)), on the formation of trihalomethanes (THMs), haloacetonitriles (HANs) and adsorbable organic halogens (AOX) in chlorinated synthetic and real waters was conducted. The pre-oxidant doses were based on their impact on natural organic matter reactivity measured by the electron donating capacity before chlorination. The influence of pH (6.5–8.1) and bromide (0–500 μg L−1) was evaluated in terms of disinfection byproduct (DBP) formation and theoretical toxicity assessment (based on THM and HAN formation). All oxidants were efficient in mitigating chlorinated DBPs, except Mn(VII) which had little impact on THM formation. O3 was generally more efficient than the other oxidants in mitigating AOX and THM formation, all pre-oxidants readily reduced the formation of HANs (>45% reduction at high dose). pH depression improved AOX mitigation by O3 and Fe(VI) but diminished Mn(VII) efficiency for all DBPs. Pre-oxidation was less efficient in mitigating brominated DBPs and generally enhanced the bromine substitution factor. Although HANs were formed at low concentrations compared to THMs, they dominated the calculated toxicity, particularly the brominated HANs. The increased dibromoacetonitrile formation after pre-oxidation was a major factor counteracting the benefits of the overall DBP mitigation. In the presence of bromide, the pre-oxidant dose should be optimized to decrease the reactivity of the matrix while controlling the toxicity induced by formation of brominated DBPs, notably brominated HANs.
中文翻译:

臭氧,二氧化氯,高铁酸盐和高锰酸盐预氧化对后氯化过程中有机消毒副产物形成的影响的比较
预氧化通常用于减轻消毒后副产物的形成。比较研究了四种预氧化剂臭氧(O 3),二氧化氯(ClO 2),高锰酸盐(Mn(VII))和高铁酸盐(Fe(VI))对三卤甲烷(THMs)形成的影响,在氯化合成水和真实水中进行了卤代乙腈(HANs)和可吸附有机卤素(AOX)的研究。预氧化剂的剂量基于它们对自然有机物反应性的影响,该影响通过氯化前的电子给体能力来衡量。pH(6.5–8.1)和溴化物(0–500μgL -1)在消毒副产物(DBP)形成和理论毒性评估(基于THM和HAN形成)方面进行了评估。除Mn(VII)对THM形成影响不大外,所有氧化剂均能有效缓解氯化DBP 。在减轻AOX和THM形成方面,O 3通常比其他氧化剂更有效,所有预氧化剂都易于减少HANs的形成(高剂量时减少> 45%)。pH降低可改善O 3和Fe(VI)的AOX缓解能力,但减少Mn(VII))所有DBP的效率。预氧化在减轻溴化DBP方面的效率较低,通常会提高溴取代因子。尽管与THM相比,HANs的形成浓度低,但它们在计算的毒性中占主导地位,尤其是溴化HANs。预氧化后增加的二溴乙腈形成是抵消总体DBP缓解的好处的主要因素。在存在溴化物的情况下,应优化预氧化剂的剂量,以降低基质的反应性,同时控制由溴化DBP(尤其是溴化HAN)的形成所引起的毒性。
更新日期:2020-06-16
中文翻译:

臭氧,二氧化氯,高铁酸盐和高锰酸盐预氧化对后氯化过程中有机消毒副产物形成的影响的比较
预氧化通常用于减轻消毒后副产物的形成。比较研究了四种预氧化剂臭氧(O 3),二氧化氯(ClO 2),高锰酸盐(Mn(VII))和高铁酸盐(Fe(VI))对三卤甲烷(THMs)形成的影响,在氯化合成水和真实水中进行了卤代乙腈(HANs)和可吸附有机卤素(AOX)的研究。预氧化剂的剂量基于它们对自然有机物反应性的影响,该影响通过氯化前的电子给体能力来衡量。pH(6.5–8.1)和溴化物(0–500μgL -1)在消毒副产物(DBP)形成和理论毒性评估(基于THM和HAN形成)方面进行了评估。除Mn(VII)对THM形成影响不大外,所有氧化剂均能有效缓解氯化DBP 。在减轻AOX和THM形成方面,O 3通常比其他氧化剂更有效,所有预氧化剂都易于减少HANs的形成(高剂量时减少> 45%)。pH降低可改善O 3和Fe(VI)的AOX缓解能力,但减少Mn(VII))所有DBP的效率。预氧化在减轻溴化DBP方面的效率较低,通常会提高溴取代因子。尽管与THM相比,HANs的形成浓度低,但它们在计算的毒性中占主导地位,尤其是溴化HANs。预氧化后增加的二溴乙腈形成是抵消总体DBP缓解的好处的主要因素。在存在溴化物的情况下,应优化预氧化剂的剂量,以降低基质的反应性,同时控制由溴化DBP(尤其是溴化HAN)的形成所引起的毒性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号