当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemically Driven and Acid-Driven Pyridine-Directed ortho-Phosphorylation of C(sp2)–H Bonds
Organometallics ( IF 2.5 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.organomet.0c00247 Tatyana V. Gryaznova 1 , Mikhail N. Khrizanforov 1 , Alina I. Levitskaya 1 , Ildar Kh.Rizvanov 1 , Marina Yu. Balakina 1 , Kamil A. Ivshin 1 , Olga N. Kataeva 1 , Yulia H. Budnikova 1
Organometallics ( IF 2.5 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.organomet.0c00247 Tatyana V. Gryaznova 1 , Mikhail N. Khrizanforov 1 , Alina I. Levitskaya 1 , Ildar Kh.Rizvanov 1 , Marina Yu. Balakina 1 , Kamil A. Ivshin 1 , Olga N. Kataeva 1 , Yulia H. Budnikova 1
Affiliation
|
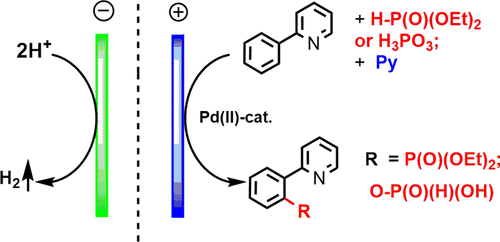
The key intermediate palladacycles (mono-, bi-, and tetranuclear) with phosphonate, acetate, and other counterions in C(sp2)–H phosphonation are analyzed in terms of their redox properties, mutual transitions, reactivity, and reaction pathways for the selective production of the desired products. It was found that, in the presence of pyridine, the reaction proceeds through a mononuclear palladacycle, which at a relatively high electrolysis potential gives the product of ortho-phosphonation of the arene with a C–P bond in good yield. Under acidic conditions, the process involves the tetrapalladium intermediate and leads to a product with a C–O–P bond. Phosphorous acid gives inorganic phosphoric derivatives of 2-phenylpyridine. The electrochemical data on the redox properties of key palladacycles 4 and 5 and their potentials and energy gaps are confirmed by DFT calculations.
中文翻译:

C(sp 2)–H键的电化学驱动和酸性驱动的吡啶定向正磷酸化
分析了C(sp 2)-H膦酸酯化过程中具有膦酸酯,乙酸根和其他抗衡离子的关键中间体Palladacycles(单核,双核和四核)的氧化还原特性,相互转变,反应性和反应途径。选择性生产所需产品。发现在吡啶的存在下,反应通过单核的palladacycle进行,该单核的palladacycle在相对较高的电解电位下可得到邻位产物带有C-P键的芳烃的膦化反应,收率很高。在酸性条件下,该过程涉及四钯中间体,并生成具有C–O–P键的产物。亚磷酸产生2-苯基吡啶的无机磷酸衍生物。通过DFT计算证实了关键Palladacycles 4和5的氧化还原性质及其电位和能隙的电化学数据。
更新日期:2020-07-13
中文翻译:

C(sp 2)–H键的电化学驱动和酸性驱动的吡啶定向正磷酸化
分析了C(sp 2)-H膦酸酯化过程中具有膦酸酯,乙酸根和其他抗衡离子的关键中间体Palladacycles(单核,双核和四核)的氧化还原特性,相互转变,反应性和反应途径。选择性生产所需产品。发现在吡啶的存在下,反应通过单核的palladacycle进行,该单核的palladacycle在相对较高的电解电位下可得到邻位产物带有C-P键的芳烃的膦化反应,收率很高。在酸性条件下,该过程涉及四钯中间体,并生成具有C–O–P键的产物。亚磷酸产生2-苯基吡啶的无机磷酸衍生物。通过DFT计算证实了关键Palladacycles 4和5的氧化还原性质及其电位和能隙的电化学数据。









































 京公网安备 11010802027423号
京公网安备 11010802027423号