当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Total Syntheses of (+)-Penostatins A and C.
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.orglett.0c01649 Jian Wang 1 , Miguel Adrián Márquez-Cadena 1 , Rongbiao Tong 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.orglett.0c01649 Jian Wang 1 , Miguel Adrián Márquez-Cadena 1 , Rongbiao Tong 1
Affiliation
|
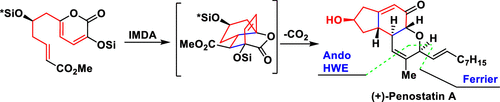
Penostatins A and C are cytotoxic natural products that show promising selective inhibitory activity against PTP1B. Here the first asymmetric total syntheses of (+)-penostatins A and C are reported. Our strategy features (i) a new method for the synthesis of 6-alkyl-3-hydroxy-2-pyrones, (ii) a cascade involving the intramolecular Diels–Alder reaction of 2-pyrone and a retro-hetero-Diels–Alder (decarboxylation) reaction, (iii) Ando–Horner–Wadsworth–Emmons olefination/lactonization, and (iv) selenoxide elimination. Our study confirmed the absolute configurations of penostatins A and C and laid the groundwork for further bioactivity studies.
中文翻译:

(+)-Penostatins A和C的不对称总合成。
Penostatins A和C是具有细胞毒性的天然产物,对PTP1B表现出有希望的选择性抑制活性。这里报道了(+)-penostatins A和C的第一个不对称的全合成。我们的策略特色(i)一种合成6-烷基-3-羟基-2-吡喃酮的新方法,(ii)级联反应涉及2-吡喃酮的分子内Diels-Alder反应和逆杂Diels-Alder反应(脱羧)反应,(iii)安多-霍纳-沃兹沃思-埃蒙斯烯化/内酯化,以及(iv)消除亚硒酸盐。我们的研究证实了戊抑素A和C的绝对构型,为进一步的生物活性研究奠定了基础。
更新日期:2020-07-02
中文翻译:

(+)-Penostatins A和C的不对称总合成。
Penostatins A和C是具有细胞毒性的天然产物,对PTP1B表现出有希望的选择性抑制活性。这里报道了(+)-penostatins A和C的第一个不对称的全合成。我们的策略特色(i)一种合成6-烷基-3-羟基-2-吡喃酮的新方法,(ii)级联反应涉及2-吡喃酮的分子内Diels-Alder反应和逆杂Diels-Alder反应(脱羧)反应,(iii)安多-霍纳-沃兹沃思-埃蒙斯烯化/内酯化,以及(iv)消除亚硒酸盐。我们的研究证实了戊抑素A和C的绝对构型,为进一步的生物活性研究奠定了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号