当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
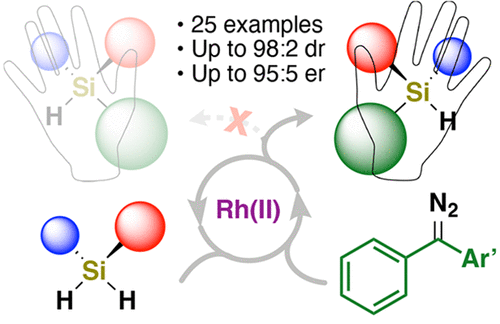
Enantioselective Si–H Insertion Reactions of Diarylcarbenes for the Synthesis of Silicon-Stereogenic Silanes
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-06-16 , DOI: 10.1021/jacs.0c04533 Jake R Jagannathan 1 , James C Fettinger 1 , Jared T Shaw 1 , Annaliese K Franz 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-06-16 , DOI: 10.1021/jacs.0c04533 Jake R Jagannathan 1 , James C Fettinger 1 , Jared T Shaw 1 , Annaliese K Franz 1
Affiliation
|
We report the first example of enantioselective, intermolecular diarylcarbene insertion into Si-H bonds for synthesis of silicon-stereogenic silanes. Dirhodium(II) carboxylates catalyze an Si-H insertion using carbenes derived from diazo compounds where selective formation of an enantioenriched silicon center is achieved using prochiral silanes. Fourteen prochiral silanes were evaluated with symmetrical and prochiral diazo reactants to produce a total of 25 novel silanes. Adding an ortho substituent on one phenyl ring of a prochiral diazo enhances enantioselectivity up to 95:5 er with yields up to 98 %. Using in situ IR spectroscopy, the impact of the off-cycle azine formation is supported based on the structural dependence for relative rates of diazo decomposition. A catalytic cycle is proposed with the Si-H insertion as the rate-determining step, supported by kinetic isotope experiments. Transformations of an enantioenriched silane derived from this method, including selective synthesis of a novel sila-indane, are demonstrated.
中文翻译:

二芳基卡宾的对映选择性 Si-H 插入反应合成硅-立体硅烷
我们报告了第一个对映选择性、分子间二芳基卡宾插入 Si-H 键以合成硅立体硅烷的例子。Dirhodium (II) 羧酸盐使用衍生自重氮化合物的卡宾催化 Si-H 插入,其中使用前手性硅烷实现对映体富集的硅中心的选择性形成。使用对称和前手性重氮反应物评估了 14 种前手性硅烷,以产生总共 25 种新型硅烷。在前手性重氮的一个苯环上添加邻位取代基可提高对映选择性高达 95:5 er,产率高达 98%。使用原位红外光谱,基于重氮分解相对速率的结构依赖性,支持非循环吖嗪形成的影响。提出了以 Si-H 插入作为速率决定步骤的催化循环,由动力学同位素实验支持。展示了源自该方法的对映体富集硅烷的转化,包括选择性合成新型硅茚满。
更新日期:2020-06-16
中文翻译:

二芳基卡宾的对映选择性 Si-H 插入反应合成硅-立体硅烷
我们报告了第一个对映选择性、分子间二芳基卡宾插入 Si-H 键以合成硅立体硅烷的例子。Dirhodium (II) 羧酸盐使用衍生自重氮化合物的卡宾催化 Si-H 插入,其中使用前手性硅烷实现对映体富集的硅中心的选择性形成。使用对称和前手性重氮反应物评估了 14 种前手性硅烷,以产生总共 25 种新型硅烷。在前手性重氮的一个苯环上添加邻位取代基可提高对映选择性高达 95:5 er,产率高达 98%。使用原位红外光谱,基于重氮分解相对速率的结构依赖性,支持非循环吖嗪形成的影响。提出了以 Si-H 插入作为速率决定步骤的催化循环,由动力学同位素实验支持。展示了源自该方法的对映体富集硅烷的转化,包括选择性合成新型硅茚满。



























 京公网安备 11010802027423号
京公网安备 11010802027423号