当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zero-overlap Fluorophores for Fluorescent Studies at Any Concentration
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-15 , DOI: 10.1021/jacs.0c02450 Ayan Dhara 1 , Tumpa Sadhukhan 1 , Edward G Sheetz 1 , Andrew H Olsson 1 , Krishnan Raghavachari 1 , Amar H Flood 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-15 , DOI: 10.1021/jacs.0c02450 Ayan Dhara 1 , Tumpa Sadhukhan 1 , Edward G Sheetz 1 , Andrew H Olsson 1 , Krishnan Raghavachari 1 , Amar H Flood 1
Affiliation
|
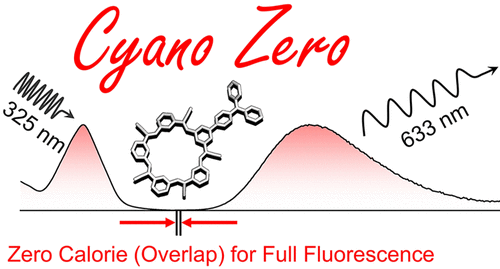
Fluorophores are powerful tools for the study of chemistry, biology and physics. However, fluorescence is severely impaired when concentrations climb above 5 M as a result of effects like self-absorption and chromatic shifts in the emitted light. Herein, we report the creation of a charge-transfer (CT) fluorophore and the discovery that its emission color seen at low concentrations is unchanged even at 5 mM, some three orders of magnitude beyond typical limits. The fluorophore is composed of a triphenylamine-substituted cyanostar macrocycle, and it exhibits a remarkable Stokes shift of 15,000 cm-1 to generate emission at 633 nm. Crucial to the performance of this fluorophore is observation that its emission spectrum shows near-zero overlap with the absorption band at 325 nm. We propose that reducing the spectral overlap to zero is a key to achieving full fluorescence across all concentrations. The triphenylamine donor and five cyanostilbene acceptor units of the macrocycle generate an emissive CT state. Unlike closely related donor-acceptor control compounds showing dual emission, the cyanostar framework inhibited emission from the second state to create a zero-overlap fluorophore. We demonstrated use of emission spectroscopy for characterization of host-guest complexation at millimolar concentrations, which are typically the exclusive domain of NMR spectroscopy. The binding of the PF6- anion generates a 2:1 sandwich complex with blue-shifted emission. Distinct from twisted intramolecular charge-transfer (TICT) states, experiment-supported density functional theory shows a 67º twist inside an acceptor unit in the CT state instead of between the donor and acceptor; it is TICT-like. Inspired by the findings we uncovered similar concentration-independent behavior from a control compound strongly suggesting this behavior may be latent to other large Stokes-shift fluorophores. We discuss strategies capable of generating zero-overlap fluorophores to enable accurate fluorescence characterization of processes across all practical concentrations.
中文翻译:

用于任何浓度荧光研究的零重叠荧光团
荧光团是化学、生物学和物理学研究的有力工具。然而,由于自吸收和发射光的色移等效应,当浓度超过 5 M 时,荧光会严重受损。在此,我们报告了电荷转移 (CT) 荧光团的创建,并发现其在低浓度下看到的发射颜色即使在 5 mM 下也没有变化,超出典型限制大约三个数量级。荧光团由三苯胺取代的氰基星大环组成,它表现出 15,000 cm-1 的显着斯托克斯位移,在 633 nm 处产生发射。该荧光团性能的关键在于观察到其发射光谱与 325 nm 处的吸收带的重叠几乎为零。我们建议将光谱重叠减少到零是在所有浓度下实现全荧光的关键。大环的三苯胺供体和五个氰基芪受体单元产生发射 CT 状态。与显示双发射的密切相关的供体-受体对照化合物不同,氰星框架抑制了第二状态的发射,以产生零重叠荧光团。我们展示了使用发射光谱来表征毫摩尔浓度的主客体络合,这通常是 NMR 光谱的专有领域。PF6- 阴离子的结合产生一个 2:1 的夹心复合物,发射蓝移。与扭曲分子内电荷转移 (TICT) 状态不同,实验支持的密度泛函理论表明,在 CT 状态下,受体单元内部而不是供体和受体之间存在 67º 扭曲;它类似于TICT。受这些发现的启发,我们从对照化合物中发现了类似的与浓度无关的行为,这强烈表明这种行为可能对其他大的斯托克斯位移荧光团是潜在的。我们讨论了能够产生零重叠荧光团的策略,以实现所有实际浓度过程的准确荧光表征。
更新日期:2020-06-15
中文翻译:

用于任何浓度荧光研究的零重叠荧光团
荧光团是化学、生物学和物理学研究的有力工具。然而,由于自吸收和发射光的色移等效应,当浓度超过 5 M 时,荧光会严重受损。在此,我们报告了电荷转移 (CT) 荧光团的创建,并发现其在低浓度下看到的发射颜色即使在 5 mM 下也没有变化,超出典型限制大约三个数量级。荧光团由三苯胺取代的氰基星大环组成,它表现出 15,000 cm-1 的显着斯托克斯位移,在 633 nm 处产生发射。该荧光团性能的关键在于观察到其发射光谱与 325 nm 处的吸收带的重叠几乎为零。我们建议将光谱重叠减少到零是在所有浓度下实现全荧光的关键。大环的三苯胺供体和五个氰基芪受体单元产生发射 CT 状态。与显示双发射的密切相关的供体-受体对照化合物不同,氰星框架抑制了第二状态的发射,以产生零重叠荧光团。我们展示了使用发射光谱来表征毫摩尔浓度的主客体络合,这通常是 NMR 光谱的专有领域。PF6- 阴离子的结合产生一个 2:1 的夹心复合物,发射蓝移。与扭曲分子内电荷转移 (TICT) 状态不同,实验支持的密度泛函理论表明,在 CT 状态下,受体单元内部而不是供体和受体之间存在 67º 扭曲;它类似于TICT。受这些发现的启发,我们从对照化合物中发现了类似的与浓度无关的行为,这强烈表明这种行为可能对其他大的斯托克斯位移荧光团是潜在的。我们讨论了能够产生零重叠荧光团的策略,以实现所有实际浓度过程的准确荧光表征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号