当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Moringin and Its Structural Analogues as Slow H2S Donors: Their Mechanisms and Bioactivity.
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.jafc.0c02358 Yuyun Lu 1 , Xingyi Wang 1 , Haoliang Pu 1 , Yi Lin 1 , Dan Li 1, 2 , Shao Quan Liu 1, 2 , Dejian Huang 1, 2
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.jafc.0c02358 Yuyun Lu 1 , Xingyi Wang 1 , Haoliang Pu 1 , Yi Lin 1 , Dan Li 1, 2 , Shao Quan Liu 1, 2 , Dejian Huang 1, 2
Affiliation
|
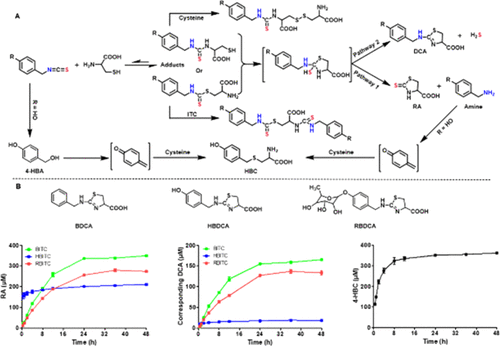
Moringin (rhamnobenzyl isothiocyanate) is a major bioactive compound in moringa seeds, which have been used as a healthy food. However, its bioactivity mechanisms are not well understood. We investigated moringin and its structurally similar analogues, including benzyl isothiocyanate and 4-hydroxylbenzyl isothiocyanate, for their hydrogen sulfide (H2S)-releasing activity triggered by cysteine. These isothiocyanates rapidly formed cysteine adducts, which underwent intramolecular cyclization followed by slowly releasing (a) organic amine and raphanusamic acid and (b) H2S and 2-carbylamino-4,5-dihydrothiazole-4-carboxylic acids. The product distributions are highly dependent on para-substituents on the phenyl group. Moringin has higher cytotoxicity to cancer cells and is a more potent anti-inflammatory agent than benzyl and hydroxybenzyl analogues, while benzyl isothiocyanate is a better antibacterial agent. Taken together, their bioactivity may not be directly related to their H2S donation activity. However, other metabolites alone do not have cytotoxicity and anti-inflammatory activity. These findings indicated that their activity may be the combination effects of different metabolites via competitive pathways as well the para-substituent groups of benzyl ITCs.
中文翻译:

辣木素及其作为慢H2S供体的结构类似物:其机理和生物活性。
辣木(辣木苄基异硫氰酸酯)是辣木种子中的主要生物活性化合物,已被用作健康食品。但是,其生物活性机制尚不十分清楚。我们研究了moringin及其结构相似的类似物,包括异硫氰酸苄酯和4-羟基异硫氰酸苄酯,其半胱氨酸引发的硫化氢(H 2 S)释放活性。这些异硫氰酸酯迅速形成半胱氨酸加合物,将其进行分子内环化,然后缓慢释放(a)有机胺和萝卜酸和(b)H 2S和2-羰基氨基-4,5-二氢噻唑-4-羧酸。产物分布高度依赖于苯基上的对位取代基。辣木素对癌细胞具有更高的细胞毒性,并且是比苄基和羟基苄基类似物更有效的抗炎剂,而异硫氰酸苄酯是一种更好的抗菌剂。两者合计,它们的生物活性可能与它们的H 2 S供体活性没有直接关系。但是,仅其他代谢物不具有细胞毒性和抗炎活性。这些发现表明,它们的活性可能是不同代谢物通过竞争途径以及苄基ITC的对位取代基的组合效应。
更新日期:2020-07-08
中文翻译:

辣木素及其作为慢H2S供体的结构类似物:其机理和生物活性。
辣木(辣木苄基异硫氰酸酯)是辣木种子中的主要生物活性化合物,已被用作健康食品。但是,其生物活性机制尚不十分清楚。我们研究了moringin及其结构相似的类似物,包括异硫氰酸苄酯和4-羟基异硫氰酸苄酯,其半胱氨酸引发的硫化氢(H 2 S)释放活性。这些异硫氰酸酯迅速形成半胱氨酸加合物,将其进行分子内环化,然后缓慢释放(a)有机胺和萝卜酸和(b)H 2S和2-羰基氨基-4,5-二氢噻唑-4-羧酸。产物分布高度依赖于苯基上的对位取代基。辣木素对癌细胞具有更高的细胞毒性,并且是比苄基和羟基苄基类似物更有效的抗炎剂,而异硫氰酸苄酯是一种更好的抗菌剂。两者合计,它们的生物活性可能与它们的H 2 S供体活性没有直接关系。但是,仅其他代谢物不具有细胞毒性和抗炎活性。这些发现表明,它们的活性可能是不同代谢物通过竞争途径以及苄基ITC的对位取代基的组合效应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号