当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparison of Layered Li(Li0.2Rh0.8)O2 and LiRhO2 upon Li Removal: Stabilizing Effect of Li Substitution.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.inorgchem.0c00970 Daria Mikhailova 1 , Sebastian Maletti 1 , Alexander Missyul 2 , Bernd Büchner 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.inorgchem.0c00970 Daria Mikhailova 1 , Sebastian Maletti 1 , Alexander Missyul 2 , Bernd Büchner 1
Affiliation
|
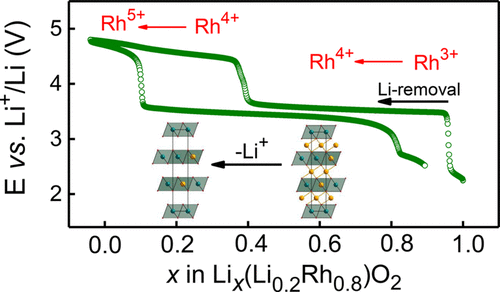
Phase transformations upon delithiation in layered oxides with the NaCrS2 structure type are widely studied for numerous combinations of 3d transition metals because of the application of LiCoO2 and its derivatives as cathode materials in rechargeable Li-ion batteries. However, complete replacement of 3d by 4d transition metals still yields phenomena never seen in compounds containing 3d metals only. In the present work, the structural evolution of Li-rich O3–Li(Li0.2Rh0.8)O2, having a mixed occupancy of 20% Li and 80% Rh in the metal–O slabs, was studied during electrochemical Li removal and insertion and compared with the isostructural stoichiometric LiRhO2. The latter compound undergoes a transformation from the layered NaCrS2 to the tunnel-like rutile–ramsdellite intergrowth structure of the γ-MnO2 type. Partial replacement of Rh by Li, in contrast, completely prevents this transition, resulting in a reversible cell expansion and shrinkage within the layered structure upon (de)lithiation. Moreover, no anomalously short Rh–O and O–O distances were observed in Lix≈0(Li0.2Rh0.8)O2 with the Rh4.75+ intermediate valence state at 4.8 V, in contrast to Lix≈0RhO2 with Rh4+ at 4.2 V, as confirmed by operando synchrotron X-ray diffraction and extended X-ray absorption fine structure studies. We believe that the difference in the Li–O and Rh–O covalency is responsible for the observed structural stabilization. The longer and more ionic Li–O bonds in the (Li,Rh)O2 layers impede the shortening of O–O distances needed for transformation to the γ-MnO2 type because of a higher negative charge on O anions connected to Li cations and the stronger electrostatic repulsion between them.
中文翻译:

去除Li时层状Li(Li0.2Rh0.8)O2和LiRhO2的比较:Li取代的稳定作用。
由于LiCoO 2及其衍生物作为可充电锂离子电池的正极材料,在3d过渡金属的多种组合中,人们广泛研究了具有NaCrS 2结构类型的层状氧化物中脱锂时的相变。但是,用4d过渡金属完全替代3d仍会产生仅在包含3d金属的化合物中从未见过的现象。在目前的工作中,研究了在电化学除锂和脱锂过程中,金属-O平板中混合占有率为20%Li和80%Rh的富Li的O3-Li(Li 0.2 Rh 0.8)O 2的结构演变。插入并与同构化学计量LiRhO 2比较。后者化合物经历从层状NaCrS变换2到γ-MnO的的隧道状金红石斜方锰矿共生结构2型。相比之下,用Li部分取代Rh可以完全防止这种转变,从而在(去)锂化时导致层状结构内可逆的单元膨胀和收缩。此外,在锂中没有观察到异常短Rho和O-O的距离X ≈0(栗0.2铑0.8)O 2与铑4.75+在4.8伏中间价态,而相比之下,李X ≈0 RHO 2与Rh 4+操作同步辐射X射线衍射和扩展的X射线吸收精细结构研究证实,在4.2 V下工作。我们认为,Li–O和Rh–O的共价差异是观察到的结构稳定的原因。的时间越长,并在(栗,铑)O多种离子型锂-O键的2层妨碍所需变换到γ-MnO的O-O的距离的缩短2因为在连接至锂阳离子ö阴离子较高负电荷型以及它们之间更强的静电排斥力。
更新日期:2020-07-06
中文翻译:

去除Li时层状Li(Li0.2Rh0.8)O2和LiRhO2的比较:Li取代的稳定作用。
由于LiCoO 2及其衍生物作为可充电锂离子电池的正极材料,在3d过渡金属的多种组合中,人们广泛研究了具有NaCrS 2结构类型的层状氧化物中脱锂时的相变。但是,用4d过渡金属完全替代3d仍会产生仅在包含3d金属的化合物中从未见过的现象。在目前的工作中,研究了在电化学除锂和脱锂过程中,金属-O平板中混合占有率为20%Li和80%Rh的富Li的O3-Li(Li 0.2 Rh 0.8)O 2的结构演变。插入并与同构化学计量LiRhO 2比较。后者化合物经历从层状NaCrS变换2到γ-MnO的的隧道状金红石斜方锰矿共生结构2型。相比之下,用Li部分取代Rh可以完全防止这种转变,从而在(去)锂化时导致层状结构内可逆的单元膨胀和收缩。此外,在锂中没有观察到异常短Rho和O-O的距离X ≈0(栗0.2铑0.8)O 2与铑4.75+在4.8伏中间价态,而相比之下,李X ≈0 RHO 2与Rh 4+操作同步辐射X射线衍射和扩展的X射线吸收精细结构研究证实,在4.2 V下工作。我们认为,Li–O和Rh–O的共价差异是观察到的结构稳定的原因。的时间越长,并在(栗,铑)O多种离子型锂-O键的2层妨碍所需变换到γ-MnO的O-O的距离的缩短2因为在连接至锂阳离子ö阴离子较高负电荷型以及它们之间更强的静电排斥力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号