当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heterologous Expression, Purification, and Functional Analysis of the Plasmodium falciparum Phosphatidylinositol 4-Kinase IIIβ.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.biochem.0c00259 Anna R Sternberg 1 , Paul D Roepe 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.biochem.0c00259 Anna R Sternberg 1 , Paul D Roepe 1
Affiliation
|
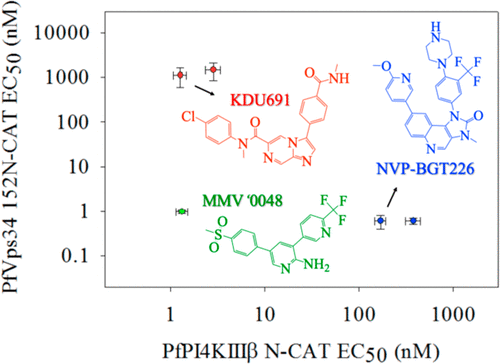
Recently, we heterologously expressed, purified, and analyzed the function of the sole Plasmodium falciparum phosphatidylinositol 3-kinase (PI3K), found that the enzyme is a “class III” or “Vps34” PI3K, and found that it is irreversibly inhibited by Fe2+-mediated covalent, nonspecific interactions with the leading antimalarial drug, dihydroartemisinin [Hassett, M. R., et al. (2017) Biochemistry56, 4335–4345]. One of several P. falciparum phosphatidylinositol 4-kinases [putative IIIβ isoform (PfPI4KIIIβ)] has generated similar interest as a druggable target; however, no validation of the mechanism of action for putative PfPI4K inhibitors has yet been possible due to the lack of purified PfPI4KIIIβ. We therefore codon optimized the pfpi4kIIIβ gene, successfully expressed the protein in yeast, and purified an N-lobe catalytic domain PfPI4KIIIβ protein. Using an enzyme-linked immunosorbent assay strategy previously perfected for analysis of PfPI3K (PfVps34), we measured the apparent initial rate, Km,app(ATP), and other enzyme characteristics and found full activity for the construct and that PfPI4KIIIβ activity is most consistent with the class IIIβ designation. Because several novel antimalarial drug candidates with different chemical scaffolds have been proposed to target PfPI4KIIIβ, we titrated enzyme inhibition for these candidates versus purified PfPI4KIIIβ and PfVps34. We also analyzed the activity versus purified PfPI4KIIIβ mutants previously expressed in P. falciparum selected for resistance to these drugs. Interestingly, we found that a putative PfPI4KIIIβ inhibitor currently in advanced trials (MMV390048; MMV ′0048) is a potent inhibitor of both PfVps34 and PfPI4KIIIβ. These data are helpful for further preclinical optimization of an exciting new class of P. falciparum PI kinase inhibitor (“PfPIKi”) antimalarial drugs.
中文翻译:

恶性疟原虫磷脂酰肌醇4-激酶IIIβ的异源表达,纯化和功能分析。
最近,我们异源表达,纯化和分析了唯一的恶性疟原虫磷脂酰肌醇3-激酶(PI3K)的功能,发现该酶是“ III类”或“ Vps34” PI3K,并发现它被铁不可逆地抑制。与领先的抗疟疾药物双氢青蒿素2+介导的共价非特异性相互作用[Hassett,MR,et al。(2017)生物化学56,4335-4345]。多次之一的恶性疟原虫磷脂酰肌醇4-激酶[公认的IIIβ同工型(PfPI4KIIIβ)]已经引起了与可药用靶标相似的兴趣。然而,由于缺乏纯化的PfPI4KIIIβ,尚未验证推定的PfPI4K抑制剂的作用机理。因此,我们密码子优化了pfpi4kIIIβ基因,在酵母中成功表达了该蛋白,并纯化了N瓣催化域PfPI4KIIIβ蛋白。使用先前完善的用于分析PfPI3K(PfVps34)的酶联免疫吸附测定策略,我们测量了表观初始速率K m,app(ATP)和其他酶的特性,发现该构建体具有完全活性,并且PfPI4KIIIβ活性与IIIβ类名称最一致。因为已经提出了几种具有不同化学支架的新型抗疟药候选物靶向PfPI4KIIIβ,所以相对于纯化的PfPI4KIIIβ和PfVps34,我们对这些候选物的酶抑制作用进行了滴定。我们还分析了针对先前在恶性疟原虫中表达的纯化的PfPI4KIIIβ突变体的活性,这些突变体被选为对这些药物具有抗药性。有趣的是,我们发现目前在高级试验中的推定的PfPI4KIIIβ抑制剂(MMV390048; MMV'0048)是PfVps34和PfPI4KIIIβ的有效抑制剂。这些数据有助于进一步开发令人兴奋的新型恶性疟原虫。 PI激酶抑制剂(“ PfPIKi”)抗疟药。
更新日期:2020-07-14
中文翻译:

恶性疟原虫磷脂酰肌醇4-激酶IIIβ的异源表达,纯化和功能分析。
最近,我们异源表达,纯化和分析了唯一的恶性疟原虫磷脂酰肌醇3-激酶(PI3K)的功能,发现该酶是“ III类”或“ Vps34” PI3K,并发现它被铁不可逆地抑制。与领先的抗疟疾药物双氢青蒿素2+介导的共价非特异性相互作用[Hassett,MR,et al。(2017)生物化学56,4335-4345]。多次之一的恶性疟原虫磷脂酰肌醇4-激酶[公认的IIIβ同工型(PfPI4KIIIβ)]已经引起了与可药用靶标相似的兴趣。然而,由于缺乏纯化的PfPI4KIIIβ,尚未验证推定的PfPI4K抑制剂的作用机理。因此,我们密码子优化了pfpi4kIIIβ基因,在酵母中成功表达了该蛋白,并纯化了N瓣催化域PfPI4KIIIβ蛋白。使用先前完善的用于分析PfPI3K(PfVps34)的酶联免疫吸附测定策略,我们测量了表观初始速率K m,app(ATP)和其他酶的特性,发现该构建体具有完全活性,并且PfPI4KIIIβ活性与IIIβ类名称最一致。因为已经提出了几种具有不同化学支架的新型抗疟药候选物靶向PfPI4KIIIβ,所以相对于纯化的PfPI4KIIIβ和PfVps34,我们对这些候选物的酶抑制作用进行了滴定。我们还分析了针对先前在恶性疟原虫中表达的纯化的PfPI4KIIIβ突变体的活性,这些突变体被选为对这些药物具有抗药性。有趣的是,我们发现目前在高级试验中的推定的PfPI4KIIIβ抑制剂(MMV390048; MMV'0048)是PfVps34和PfPI4KIIIβ的有效抑制剂。这些数据有助于进一步开发令人兴奋的新型恶性疟原虫。 PI激酶抑制剂(“ PfPIKi”)抗疟药。











































 京公网安备 11010802027423号
京公网安备 11010802027423号