当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Probing the Enzymatic Activity of Individual Biocatalytic fd-Viral Particles by Electrochemical-Atomic Force Microscopy
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-06-15 , DOI: 10.1021/acscatal.0c01920 Telmo O. Paiva 1 , Kristian Torbensen 1 , Anisha N. Patel 1 , Agnès Anne 1 , Arnaud Chovin 1 , Christophe Demaille 1 , Laure Bataille 2 , Thierry Michon 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-06-15 , DOI: 10.1021/acscatal.0c01920 Telmo O. Paiva 1 , Kristian Torbensen 1 , Anisha N. Patel 1 , Agnès Anne 1 , Arnaud Chovin 1 , Christophe Demaille 1 , Laure Bataille 2 , Thierry Michon 2
Affiliation
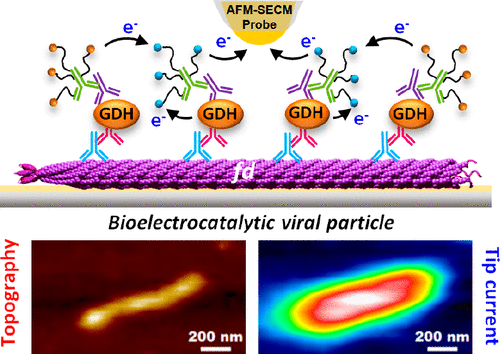
|
Surface-immobilized fd bacteriophage particles are used as scaffolds to coassemble the redox enzyme quinoprotein glucose dehydrogenase, PQQ-GDH, and its cosubstrate, PEG-tethered ferrocene. Individual decorated fd phages are visualized and simultaneously functionally interrogated by Mt/AFM-SECM microscopy, an in situ local probe correlative imaging technique, combining atomic force (AFM) and electrochemical (SECM) microscopy in a mediator tethered (Mt) configuration. The statistical distribution of catalytic activity across the fd population is resolved, and the correlation between the functional properties of the phages and their actual dimensions is assessed. Moreover, achievement of subparticle resolution allows the enzymatic activity of individual viruses to be spatially mapped, revealing a highly active region located in the middle of the filamentous fd-scaffold. Quantitative modeling shows that this “catalytic hot-spot” arises from the interplay between charge transport by electron hopping between ferrocene moieties along the viral particles and enzymatic catalysis. The developed model also enables complete analysis of GDH kinetics at the single bioscaffold scale, revealing differences in the functional behavior of the biocatalytic viral particles when addressed at the ensemble or at the single-particle scale.
中文翻译:

探索个人生物催化的酶活性FD通过电化学原子力显微镜-Viral颗粒
将表面固定的fd噬菌体颗粒用作支架,以共组装氧化还原酶奎蛋白葡萄糖脱氢酶PQQ-GDH及其共基质PEG系二茂铁。单个修饰的fd噬菌体可通过Mt / AFM-SECM显微镜(一种原位局部探针相关成像技术)可视化,并在功能上同时进行询问,该技术将原子力(AFM)和电化学(SECM)显微镜结合为介体束缚(Mt)。FD的催化活性的统计分布解决种群数量,评估噬菌体功能特性与其实际大小之间的相关性。此外,亚粒子分辨率的成就允许个别病毒的酶活性被空间映射,揭示位于丝状的中间的高活性区域FD -scaffold。定量建模表明,这种“催化热点”是由于二茂铁部分沿着病毒颗粒之间的电子跳跃和电子催化之间的相互作用而产生的。所开发的模型还能够在单个生物支架规模上完整分析GDH动力学,揭示了以整体或单个颗粒规模处理时生物催化病毒颗粒功能行为的差异。
更新日期:2020-07-17
中文翻译:

探索个人生物催化的酶活性FD通过电化学原子力显微镜-Viral颗粒
将表面固定的fd噬菌体颗粒用作支架,以共组装氧化还原酶奎蛋白葡萄糖脱氢酶PQQ-GDH及其共基质PEG系二茂铁。单个修饰的fd噬菌体可通过Mt / AFM-SECM显微镜(一种原位局部探针相关成像技术)可视化,并在功能上同时进行询问,该技术将原子力(AFM)和电化学(SECM)显微镜结合为介体束缚(Mt)。FD的催化活性的统计分布解决种群数量,评估噬菌体功能特性与其实际大小之间的相关性。此外,亚粒子分辨率的成就允许个别病毒的酶活性被空间映射,揭示位于丝状的中间的高活性区域FD -scaffold。定量建模表明,这种“催化热点”是由于二茂铁部分沿着病毒颗粒之间的电子跳跃和电子催化之间的相互作用而产生的。所开发的模型还能够在单个生物支架规模上完整分析GDH动力学,揭示了以整体或单个颗粒规模处理时生物催化病毒颗粒功能行为的差异。



























 京公网安备 11010802027423号
京公网安备 11010802027423号