当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual-Ligand-Enabled Ir(III)-Catalyzed Enantioselective C–H Amidation for the Synthesis of Chiral Sulfoxides
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-15 , DOI: 10.1021/acscatal.0c02109 Wentan Liu 1 , Wu Yang 1 , Jiefeng Zhu 1 , Yonghong Guo 1 , Na Wang 1 , Jie Ke 1 , Peiyuan Yu 1 , Chuan He 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-15 , DOI: 10.1021/acscatal.0c02109 Wentan Liu 1 , Wu Yang 1 , Jiefeng Zhu 1 , Yonghong Guo 1 , Na Wang 1 , Jie Ke 1 , Peiyuan Yu 1 , Chuan He 1
Affiliation
|
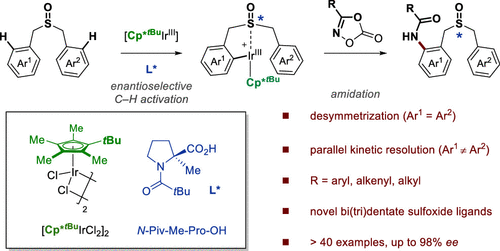
A rational designed Ir(III)-catalyzed enantioselective C–H amidation of dibenzyl sulfoxides through desymmetrization and parallel kinetic resolution is demonstrated. An Ir(III) complex equipped with a t-butyl cyclopentadienyl ligand and paired with a modified chiral proline enables the highly enantioselective sulfoxide-steered C–H bond activation, providing an efficient and straightforward way to construct sulfur chiral centers. A wide range of dibenzyl sulfoxides and dioxazolones are compatible with this process, giving access to a variety of highly functionalized sulfoxide compounds with synthetically attractive amide substitution groups in good yields and enantioselectivities. Moreover, the flexible derivatization of the amidated sulfoxide was elaborated, providing various types of chiral sulfoxide scaffolds that would be potentially useful in asymmetric catalysis as chiral bidentate and tridentate ligands.
中文翻译:

双配体使能的Ir(III)催化的对映选择性CH酰胺化反应,用于手性亚砜的合成
通过脱对称和平行动力学拆分,证明了合理设计的Ir(III)催化的二苄基亚砜的Ir(III)对映选择性C–H酰胺化。配备有t的Ir(III)络合物丁基丁基环戊二烯基配体与修饰的手性脯氨酸配对可实现高度对映选择性的亚砜导向的C–H键活化,为构建硫手性中心提供了一种有效而直接的方法。各种各样的二苄亚砜和二恶唑酮都与此方法兼容,从而以高收率和对映选择性获得了具有合成上有吸引力的酰胺取代基的各种高度官能化的亚砜化合物。此外,对酰胺化亚砜的柔性衍生进行了详细说明,从而提供了各种类型的手性亚砜骨架,它们可能作为手性二齿和三齿配体用于不对称催化。
更新日期:2020-07-02
中文翻译:

双配体使能的Ir(III)催化的对映选择性CH酰胺化反应,用于手性亚砜的合成
通过脱对称和平行动力学拆分,证明了合理设计的Ir(III)催化的二苄基亚砜的Ir(III)对映选择性C–H酰胺化。配备有t的Ir(III)络合物丁基丁基环戊二烯基配体与修饰的手性脯氨酸配对可实现高度对映选择性的亚砜导向的C–H键活化,为构建硫手性中心提供了一种有效而直接的方法。各种各样的二苄亚砜和二恶唑酮都与此方法兼容,从而以高收率和对映选择性获得了具有合成上有吸引力的酰胺取代基的各种高度官能化的亚砜化合物。此外,对酰胺化亚砜的柔性衍生进行了详细说明,从而提供了各种类型的手性亚砜骨架,它们可能作为手性二齿和三齿配体用于不对称催化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号