当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Accelerated Calcium Phosphate Mineralization by Peptides with Adjacent Oppositely Charged Residues
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-06-15 , DOI: 10.1021/acsbiomaterials.0c00194 Mustafa Gungormus 1, 2 , Mahmut Sertac Ozdogan 3 , Sinan Yasin Ertem 3 , Fatih Tulumbaci 3 , Halil Kara 4 , Metin Orhan 3
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-06-15 , DOI: 10.1021/acsbiomaterials.0c00194 Mustafa Gungormus 1, 2 , Mahmut Sertac Ozdogan 3 , Sinan Yasin Ertem 3 , Fatih Tulumbaci 3 , Halil Kara 4 , Metin Orhan 3
Affiliation
|
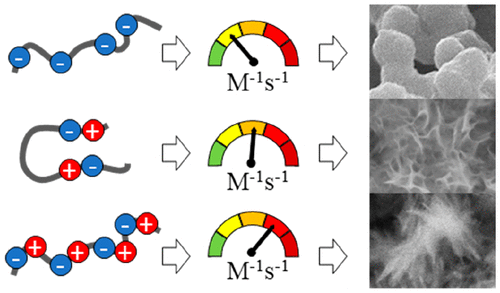
Calcium phosphate mineralizing peptides are of special importance for dental and orthopedic applications, such as caries remineralization and improved osteointegration. Uncovering the mechanism of action for such peptides is an ongoing challenge with the aim of a better fundamental understanding of biomineralization processes and developing optimized peptides for clinical use. It has recently been reported that “adjacent oppositely charged residue” motifs are found abundantly in cation binding, inorganic surface binding, or biomineralization-related proteins and may play a key role in the biomineralization events. Despite their medical importance, the role of these motifs has not yet been investigated on calcium phosphate mineral systems. To investigate this, we have designed peptides with different structural properties and different numbers of adjacent oppositely charged residues. We have evaluated their effects on in vitro calcium phosphate mineralization kinetics and mineral properties. The kinetics of the mineralization increased proportionally with an increasing number of adjacent oppositely charged residues. Two peptides with relatively high structural stability and two adjacent oppositely charged residues resulted in faster mineralization and more crystalline mineral compared to a peptide with a higher structural degree of freedom that contained only acidic residues. The fastest mineralization and the highest mineral crystallinity were obtained with a peptide containing the highest number of adjacent oppositely charged residues and highest structural degree of freedom. Our findings and observations from previously identified natural or designed peptides indicate that, in addition to structural instability, adjacent oppositely charged residues play a role in the cation binding, inorganic surface binding, and biomineralization of peptides and require further investigation. Lastly, the peptide identified in this study is an agent with potential medical applications involving the treatment of mineralized tissues.
中文翻译:

带有相邻相反电荷残基的肽加速磷酸钙的矿化
磷酸钙矿化肽对于牙科和整形外科应用特别重要,例如龋齿再矿化和改善的骨整合。揭示此类肽的作用机理是一项持续的挑战,目的是更好地了解生物矿化过程并开发出可用于临床的优化肽。最近有报道说,在阳离子结合,无机表面结合或与生物矿化有关的蛋白质中大量发现“相邻的带相反电荷的残基”基序,并可能在生物矿化事件中起关键作用。尽管它们具有医学重要性,但尚未在磷酸钙矿物质系统上研究这些基序的作用。为了对此进行调查,我们设计了具有不同结构特性和不同数目的相邻带相反电荷残基的肽。我们已经评估了它们对体外磷酸钙的矿化动力学和矿物特性。矿化动力学随相邻带相反电荷的残渣数量的增加成比例地增加。与仅包含酸性残基的具有较高结构自由度的肽相比,具有相对较高的结构稳定性的两个肽和两个相邻的带相反电荷的残基导致更快的矿化和更多的结晶矿物。用含有最多数量的相邻带相反电荷的残基和最高结构自由度的肽可获得最快的矿化和最高的矿物结晶度。我们对先前鉴定的天然或设计肽段的发现和观察表明,除了结构不稳定性之外,相邻的带相反电荷的残基在肽的阳离子结合,无机表面结合和生物矿化中起作用,需要进一步研究。最后,在这项研究中鉴定出的肽是一种具有潜在医学应用的药物,涉及治疗矿化组织。
更新日期:2020-07-13
中文翻译:

带有相邻相反电荷残基的肽加速磷酸钙的矿化
磷酸钙矿化肽对于牙科和整形外科应用特别重要,例如龋齿再矿化和改善的骨整合。揭示此类肽的作用机理是一项持续的挑战,目的是更好地了解生物矿化过程并开发出可用于临床的优化肽。最近有报道说,在阳离子结合,无机表面结合或与生物矿化有关的蛋白质中大量发现“相邻的带相反电荷的残基”基序,并可能在生物矿化事件中起关键作用。尽管它们具有医学重要性,但尚未在磷酸钙矿物质系统上研究这些基序的作用。为了对此进行调查,我们设计了具有不同结构特性和不同数目的相邻带相反电荷残基的肽。我们已经评估了它们对体外磷酸钙的矿化动力学和矿物特性。矿化动力学随相邻带相反电荷的残渣数量的增加成比例地增加。与仅包含酸性残基的具有较高结构自由度的肽相比,具有相对较高的结构稳定性的两个肽和两个相邻的带相反电荷的残基导致更快的矿化和更多的结晶矿物。用含有最多数量的相邻带相反电荷的残基和最高结构自由度的肽可获得最快的矿化和最高的矿物结晶度。我们对先前鉴定的天然或设计肽段的发现和观察表明,除了结构不稳定性之外,相邻的带相反电荷的残基在肽的阳离子结合,无机表面结合和生物矿化中起作用,需要进一步研究。最后,在这项研究中鉴定出的肽是一种具有潜在医学应用的药物,涉及治疗矿化组织。










































 京公网安备 11010802027423号
京公网安备 11010802027423号