当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Photodynamic Therapy Using the Apohemoglobin-Haptoglobin Complex as a Carrier of Aluminum Phthalocyanine
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-06-15 , DOI: 10.1021/acsabm.0c00450 Ivan S Pires 1 , Quintin T O'Boyle 1 , Carlos J Munoz 2 , Chintan Savla 1 , Pedro Cabrales 2 , Andre F Palmer 1
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-06-15 , DOI: 10.1021/acsabm.0c00450 Ivan S Pires 1 , Quintin T O'Boyle 1 , Carlos J Munoz 2 , Chintan Savla 1 , Pedro Cabrales 2 , Andre F Palmer 1
Affiliation
|
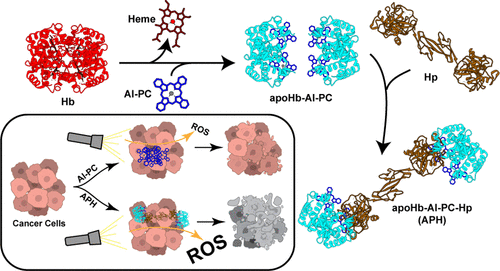
Photodynamic therapy (PDT) has been shown to effectively treat cancer by producing cytotoxic reactive oxygen species via excitation of photosensitizer (PS). However, most PS lack tumor cell specificity, possess poor aqueous solubility, and cause systemic photosensitivity. Removing heme from hemoglobin (Hb) yields an apoprotein called apohemoglobin (apoHb) with a vacant heme-binding pocket that can efficiently bind to hydrophobic molecules such as PS. In this study, the PS aluminum phthalocyanine (Al-PC) was bound to the apoHb-haptoglobin (apoHb-Hp) protein complex, forming an apoHb-Al-PC-Hp (APH) complex. The reaction of Al-PC with apoHb prevented Al-PC aggregation in aqueous solution, retaining the characteristic spectral properties of Al-PC. The stability of apoHb-Al-PC was enhanced via binding with Hp to form the APH complex, which allowed for repeated Al-PC additions to maximize Al-PC encapsulation. The final APH product had 65% of the active heme-binding sites of apoHb bound to Al-PC and a hydrodynamic diameter of 18 nm that could potentially reduce extravasation of the molecule through the blood vessel wall and prevent kidney accumulation of Al-PC. Furthermore, more than 80% of APH’s absorbance spectra were retained when incubated for over a day in plasma at 37 °C. Heme displacement assays confirmed that Al-PC was bound within the heme-binding pocket of apoHb and binding specificity was demonstrated by ineffective Al-PC binding to human serum albumin, Hp, or Hb. In vitro studies confirmed enhanced singlet oxygen generation of APH over Al-PC in aqueous solution and demonstrated effective PDT on human and murine cancer cells. Taken together, this study provides a method to produce APH for enhanced PDT via improved PS solubility and potential targeted therapy via uptake by CD163+ macrophages and monocytes in the tumor (i.e., tumor-associated macrophages). Moreover, this scalable method for site-specific encapsulation of Al-PC into apoHb and apoHb-Hp may be used for other hydrophobic therapeutic agents.
中文翻译:

使用脱氧血红蛋白-触珠蛋白复合物作为铝酞菁载体的增强光动力疗法
光动力疗法 (PDT) 已被证明可以通过激发光敏剂 (PS) 产生细胞毒性活性氧来有效治疗癌症。然而,大多数PS缺乏肿瘤细胞特异性,水溶性差,并引起全身光敏性。从血红蛋白 (Hb) 中去除血红素会产生一种称为脱辅基血红蛋白 (apoHb) 的脱辅基蛋白,该脱辅基蛋白具有一个空的血红素结合口袋,可以有效地与 PS 等疏水分子结合。在这项研究中,PS 铝酞菁 (Al-PC) 与 apoHb-触珠蛋白 (apoHb-Hp) 蛋白复合物结合,形成 apoHb-Al-PC-Hp (APH) 复合物。Al-PC 与 apoHb 的反应阻止了 Al-PC 在水溶液中的聚集,保留了 Al-PC 的特征光谱特性。apoHb-Al-PC 的稳定性通过与 Hp 结合形成 APH 复合物而增强,这允许重复添加 Al-PC 以最大化 Al-PC 封装。最终的 APH 产品有 65% 的 apoHb 活性血红素结合位点与 Al-PC 结合,流体动力学直径为 18 nm,这可能会减少分子通过血管壁的外渗并防止 Al-PC 的肾脏积聚。此外,在 37 °C 的血浆中孵育一天以上时,超过 80% 的 APH 吸收光谱得以保留。血红素置换测定证实,Al-PC 结合在 apoHb 的血红素结合口袋内,结合特异性通过无效的 Al-PC 与人血清白蛋白、Hp 或 Hb 结合来证明。最终的 APH 产品有 65% 的 apoHb 活性血红素结合位点与 Al-PC 结合,流体动力学直径为 18 nm,这可能会减少分子通过血管壁的外渗并防止 Al-PC 的肾脏积聚。此外,在 37 °C 的血浆中孵育一天以上时,超过 80% 的 APH 吸收光谱得以保留。血红素置换测定证实,Al-PC 结合在 apoHb 的血红素结合口袋内,结合特异性通过无效的 Al-PC 与人血清白蛋白、Hp 或 Hb 结合来证明。最终的 APH 产品有 65% 的 apoHb 活性血红素结合位点与 Al-PC 结合,流体动力学直径为 18 nm,这可能会减少分子通过血管壁的外渗并防止 Al-PC 的肾脏积聚。此外,在 37 °C 的血浆中孵育一天以上时,超过 80% 的 APH 吸收光谱得以保留。血红素置换测定证实,Al-PC 结合在 apoHb 的血红素结合口袋内,结合特异性通过无效的 Al-PC 与人血清白蛋白、Hp 或 Hb 结合来证明。体外研究证实,水溶液中 APH 的单线态氧生成比 Al-PC 更强,并证明了对人和小鼠癌细胞的有效 PDT。总之,本研究提供了一种通过提高 PS 溶解度和通过肿瘤中的 CD163+ 巨噬细胞和单核细胞(即肿瘤相关巨噬细胞)摄取的潜在靶向治疗来产生用于增强 PDT 的 APH 的方法。此外,这种用于将 Al-PC 特定位点封装到 apoHb 和 apoHb-Hp 中的可扩展方法可用于其他疏水性治疗剂。
更新日期:2020-07-20
中文翻译:

使用脱氧血红蛋白-触珠蛋白复合物作为铝酞菁载体的增强光动力疗法
光动力疗法 (PDT) 已被证明可以通过激发光敏剂 (PS) 产生细胞毒性活性氧来有效治疗癌症。然而,大多数PS缺乏肿瘤细胞特异性,水溶性差,并引起全身光敏性。从血红蛋白 (Hb) 中去除血红素会产生一种称为脱辅基血红蛋白 (apoHb) 的脱辅基蛋白,该脱辅基蛋白具有一个空的血红素结合口袋,可以有效地与 PS 等疏水分子结合。在这项研究中,PS 铝酞菁 (Al-PC) 与 apoHb-触珠蛋白 (apoHb-Hp) 蛋白复合物结合,形成 apoHb-Al-PC-Hp (APH) 复合物。Al-PC 与 apoHb 的反应阻止了 Al-PC 在水溶液中的聚集,保留了 Al-PC 的特征光谱特性。apoHb-Al-PC 的稳定性通过与 Hp 结合形成 APH 复合物而增强,这允许重复添加 Al-PC 以最大化 Al-PC 封装。最终的 APH 产品有 65% 的 apoHb 活性血红素结合位点与 Al-PC 结合,流体动力学直径为 18 nm,这可能会减少分子通过血管壁的外渗并防止 Al-PC 的肾脏积聚。此外,在 37 °C 的血浆中孵育一天以上时,超过 80% 的 APH 吸收光谱得以保留。血红素置换测定证实,Al-PC 结合在 apoHb 的血红素结合口袋内,结合特异性通过无效的 Al-PC 与人血清白蛋白、Hp 或 Hb 结合来证明。最终的 APH 产品有 65% 的 apoHb 活性血红素结合位点与 Al-PC 结合,流体动力学直径为 18 nm,这可能会减少分子通过血管壁的外渗并防止 Al-PC 的肾脏积聚。此外,在 37 °C 的血浆中孵育一天以上时,超过 80% 的 APH 吸收光谱得以保留。血红素置换测定证实,Al-PC 结合在 apoHb 的血红素结合口袋内,结合特异性通过无效的 Al-PC 与人血清白蛋白、Hp 或 Hb 结合来证明。最终的 APH 产品有 65% 的 apoHb 活性血红素结合位点与 Al-PC 结合,流体动力学直径为 18 nm,这可能会减少分子通过血管壁的外渗并防止 Al-PC 的肾脏积聚。此外,在 37 °C 的血浆中孵育一天以上时,超过 80% 的 APH 吸收光谱得以保留。血红素置换测定证实,Al-PC 结合在 apoHb 的血红素结合口袋内,结合特异性通过无效的 Al-PC 与人血清白蛋白、Hp 或 Hb 结合来证明。体外研究证实,水溶液中 APH 的单线态氧生成比 Al-PC 更强,并证明了对人和小鼠癌细胞的有效 PDT。总之,本研究提供了一种通过提高 PS 溶解度和通过肿瘤中的 CD163+ 巨噬细胞和单核细胞(即肿瘤相关巨噬细胞)摄取的潜在靶向治疗来产生用于增强 PDT 的 APH 的方法。此外,这种用于将 Al-PC 特定位点封装到 apoHb 和 apoHb-Hp 中的可扩展方法可用于其他疏水性治疗剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号