Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Neuropathy-associated histidyl-tRNA synthetase variants attenuate protein synthesis in vitro and disrupt axon outgrowth in developing zebrafish.
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-06-15 , DOI: 10.1111/febs.15449 Patrick Mullen 1 , Jamie A Abbott 1 , Theresa Wellman 2 , Mahafuza Aktar 1 , Christian Fjeld 1 , Borries Demeler 3 , Alicia M Ebert 4 , Christopher S Francklyn 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-06-15 , DOI: 10.1111/febs.15449 Patrick Mullen 1 , Jamie A Abbott 1 , Theresa Wellman 2 , Mahafuza Aktar 1 , Christian Fjeld 1 , Borries Demeler 3 , Alicia M Ebert 4 , Christopher S Francklyn 1
Affiliation
|
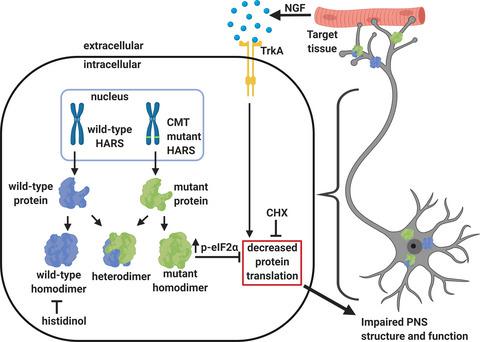
Charcot‐Marie‐Tooth disease (CMT) encompasses a set of genetically and clinically heterogeneous neuropathies characterized by length‐dependent dysfunction of the peripheral nervous system. Mutations in over 80 diverse genes are associated with CMT, and aminoacyl‐tRNA synthetases (ARS) constitute a large gene family implicated in the disease. Despite considerable efforts to elucidate the mechanistic link between ARS mutations and the CMT phenotype, the molecular basis of the pathology is unknown. In this work, we investigated the impact of three CMT‐associated substitutions (V155G, Y330C, and R137Q) in the cytoplasmic histidyl‐tRNA synthetase (HARS1) on neurite outgrowth and peripheral nervous system development. The model systems for this work included a nerve growth factor‐stimulated neurite outgrowth model in rat pheochromocytoma cells (PC12), and a zebrafish line with GFP/red fluorescent protein reporters of sensory and motor neuron development. The expression of CMT‐HARS1 mutations led to attenuation of protein synthesis and increased phosphorylation of eIF2α in PC12 cells and was accompanied by impaired neurite and axon outgrowth in both models. Notably, these effects were phenocopied by histidinol, a HARS1 inhibitor, and cycloheximide, a protein synthesis inhibitor. The mutant proteins also formed heterodimers with wild‐type HARS1, raising the possibility that CMT‐HARS1 mutations cause disease through a dominant‐negative mechanism. Overall, these findings support the hypothesis that CMT‐HARS1 alleles exert their toxic effect in a neuronal context, and lead to dysregulated protein synthesis. These studies demonstrate the value of zebrafish as a model for studying mutant alleles associated with CMT, and for characterizing the processes that lead to peripheral nervous system dysfunction.
中文翻译:

神经病相关的组氨酰-tRNA 合成酶变体会减弱体外蛋白质合成,并破坏发育中斑马鱼的轴突生长。
夏科-玛丽-图思病 (CMT) 包括一系列遗传和临床异质性神经病,其特征是周围神经系统的长度依赖性功能障碍。 80 多个不同基因的突变与 CMT 相关,氨酰基-tRNA 合成酶 (ARS) 构成了与该疾病相关的一个大基因家族。尽管人们付出了大量努力来阐明 ARS 突变与 CMT 表型之间的机制联系,但其病理学的分子基础尚不清楚。在这项工作中,我们研究了细胞质组氨酰-tRNA 合成酶 (HARS1) 中三个 CMT 相关取代(V155G、Y330C 和 R137Q)对神经突生长和周围神经系统发育的影响。这项工作的模型系统包括大鼠嗜铬细胞瘤细胞 (PC12) 中神经生长因子刺激的神经突生长模型,以及带有感觉和运动神经元发育的 GFP/红色荧光蛋白报告基因的斑马鱼系。 CMT-HARS1突变的表达导致PC12细胞中蛋白质合成减弱和eIF2α磷酸化增加,并且在两种模型中都伴随着神经突和轴突生长受损。值得注意的是,这些作用可通过组氨醇(一种 HARS1 抑制剂)和放线菌酮(一种蛋白质合成抑制剂)进行表型复制。突变蛋白还与野生型 HARS1 形成异二聚体,这增加了 CMT-HARS1 突变通过显性失活机制导致疾病的可能性。总的来说,这些发现支持这样的假设:CMT- HARS1等位基因在神经元环境中发挥毒性作用,并导致蛋白质合成失调。 这些研究证明了斑马鱼作为研究与 CMT 相关的突变等位基因以及表征导致周围神经系统功能障碍的过程的模型的价值。
更新日期:2020-06-15
中文翻译:

神经病相关的组氨酰-tRNA 合成酶变体会减弱体外蛋白质合成,并破坏发育中斑马鱼的轴突生长。
夏科-玛丽-图思病 (CMT) 包括一系列遗传和临床异质性神经病,其特征是周围神经系统的长度依赖性功能障碍。 80 多个不同基因的突变与 CMT 相关,氨酰基-tRNA 合成酶 (ARS) 构成了与该疾病相关的一个大基因家族。尽管人们付出了大量努力来阐明 ARS 突变与 CMT 表型之间的机制联系,但其病理学的分子基础尚不清楚。在这项工作中,我们研究了细胞质组氨酰-tRNA 合成酶 (HARS1) 中三个 CMT 相关取代(V155G、Y330C 和 R137Q)对神经突生长和周围神经系统发育的影响。这项工作的模型系统包括大鼠嗜铬细胞瘤细胞 (PC12) 中神经生长因子刺激的神经突生长模型,以及带有感觉和运动神经元发育的 GFP/红色荧光蛋白报告基因的斑马鱼系。 CMT-HARS1突变的表达导致PC12细胞中蛋白质合成减弱和eIF2α磷酸化增加,并且在两种模型中都伴随着神经突和轴突生长受损。值得注意的是,这些作用可通过组氨醇(一种 HARS1 抑制剂)和放线菌酮(一种蛋白质合成抑制剂)进行表型复制。突变蛋白还与野生型 HARS1 形成异二聚体,这增加了 CMT-HARS1 突变通过显性失活机制导致疾病的可能性。总的来说,这些发现支持这样的假设:CMT- HARS1等位基因在神经元环境中发挥毒性作用,并导致蛋白质合成失调。 这些研究证明了斑马鱼作为研究与 CMT 相关的突变等位基因以及表征导致周围神经系统功能障碍的过程的模型的价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号