Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Plant protein phosphatases: What do we know about their mechanism of action?
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-06-15 , DOI: 10.1111/febs.15454 Malathi Bheri 1 , Swati Mahiwal 1 , Sibaji K Sanyal 1 , Girdhar K Pandey 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-06-15 , DOI: 10.1111/febs.15454 Malathi Bheri 1 , Swati Mahiwal 1 , Sibaji K Sanyal 1 , Girdhar K Pandey 1
Affiliation
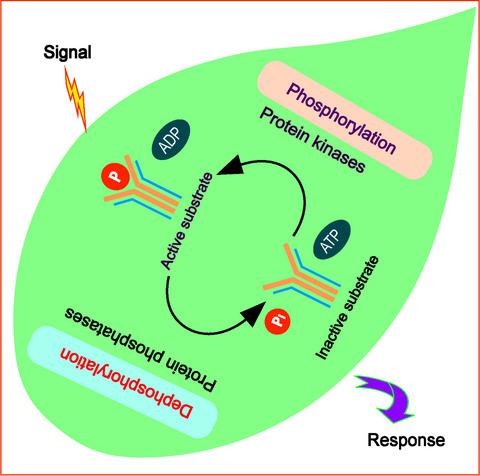
|
Protein phosphorylation is a major reversible post‐translational modification. Protein phosphatases function as ‘critical regulators’ in signaling networks through dephosphorylation of proteins, which have been phosphorylated by protein kinases. A large understanding of their working has been sourced from animal systems rather than the plant or the prokaryotic systems. The eukaryotic protein phosphatases include phosphoprotein phosphatases (PPP), metallo‐dependent protein phosphatases (PPM), protein tyrosine (Tyr) phosphatases (PTP), and aspartate (Asp)‐dependent phosphatases. The PPP and PPM families are serine(Ser)/threonine(Thr)‐specific phosphatases (STPs), while PTP family is Tyr specific. Dual‐specificity phosphatases (DsPTPs/DSPs) dephosphorylate Ser, Thr, and Tyr residues. PTPs lack sequence homology with STPs, indicating a difference in catalytic mechanisms, while the PPP and PPM families share a similar structural fold indicating a common catalytic mechanism. The catalytic cysteine (Cys) residue in the conserved HCX5R active site motif of the PTPs acts as a nucleophile during hydrolysis. The PPP members require metal ions, which coordinate the phosphate group of the substrate, followed by a nucleophilic attack by a water molecule and hydrolysis. The variable holoenzyme assembly of protein phosphatase(s) and the overlap with other post‐translational modifications like acetylation and ubiquitination add to their complexity. Though their functional characterization is extensively reported in plants, the mechanistic nature of their action is still being explored by researchers. In this review, we exclusively overview the plant protein phosphatases with an emphasis on their mechanistic action as well as structural characteristics.
中文翻译:

植物蛋白磷酸酶:我们对它们的作用机理了解多少?
蛋白质磷酸化是主要的可逆翻译后修饰。蛋白质磷酸酶通过蛋白质的去磷酸化在信号网络中充当“关键调节剂”,而蛋白质已被蛋白质激酶磷酸化。对它们工作原理的广泛了解来自动物系统,而不是植物或原核系统。真核蛋白质磷酸酶包括磷酸蛋白质磷酸酶(PPP),金属依赖性蛋白质磷酸酶(PPM),蛋白质酪氨酸(Tyr)磷酸酶(PTP)和天冬氨酸(Asp)依赖性磷酸酶。PPP和PPM家族是丝氨酸(Ser)/苏氨酸(Thr)特异的磷酸酶(STP),而PTP家族是Tyr特异的。双特异性磷酸酶(DsPTP / DSP)使Ser,Thr和Tyr残基脱磷酸。PTP与STP缺乏序列同源性,表示催化机制不同,而PPP和PPM家族具有相似的结构折叠,说明共有的催化机制。保守的HCX中的催化半胱氨酸(Cys)残留PTP的5 R活性位点基序在水解过程中充当亲核试剂。PPP成员需要金属离子,这些离子与底物的磷酸根基团配位,随后被水分子进行亲核攻击并发生水解。蛋白磷酸酶的可变全酶组装以及与其他翻译后修饰(如乙酰化和泛素化)的重叠增加了它们的复杂性。尽管在植物中广泛报道了它们的功能特性,但研究人员仍在探索其作用的机械性质。在这篇综述中,我们专门概述了植物蛋白磷酸酶,重点是其机械作用以及结构特征。
更新日期:2020-06-15
中文翻译:

植物蛋白磷酸酶:我们对它们的作用机理了解多少?
蛋白质磷酸化是主要的可逆翻译后修饰。蛋白质磷酸酶通过蛋白质的去磷酸化在信号网络中充当“关键调节剂”,而蛋白质已被蛋白质激酶磷酸化。对它们工作原理的广泛了解来自动物系统,而不是植物或原核系统。真核蛋白质磷酸酶包括磷酸蛋白质磷酸酶(PPP),金属依赖性蛋白质磷酸酶(PPM),蛋白质酪氨酸(Tyr)磷酸酶(PTP)和天冬氨酸(Asp)依赖性磷酸酶。PPP和PPM家族是丝氨酸(Ser)/苏氨酸(Thr)特异的磷酸酶(STP),而PTP家族是Tyr特异的。双特异性磷酸酶(DsPTP / DSP)使Ser,Thr和Tyr残基脱磷酸。PTP与STP缺乏序列同源性,表示催化机制不同,而PPP和PPM家族具有相似的结构折叠,说明共有的催化机制。保守的HCX中的催化半胱氨酸(Cys)残留PTP的5 R活性位点基序在水解过程中充当亲核试剂。PPP成员需要金属离子,这些离子与底物的磷酸根基团配位,随后被水分子进行亲核攻击并发生水解。蛋白磷酸酶的可变全酶组装以及与其他翻译后修饰(如乙酰化和泛素化)的重叠增加了它们的复杂性。尽管在植物中广泛报道了它们的功能特性,但研究人员仍在探索其作用的机械性质。在这篇综述中,我们专门概述了植物蛋白磷酸酶,重点是其机械作用以及结构特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号