当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel‐Catalyzed Dicarbofunctionalization of Alkenes†
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-06-16 , DOI: 10.1002/cjoc.202000224 Yun‐Cheng Luo 1 , Chang Xu 1 , Xingang Zhang 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-06-16 , DOI: 10.1002/cjoc.202000224 Yun‐Cheng Luo 1 , Chang Xu 1 , Xingang Zhang 1
Affiliation
|
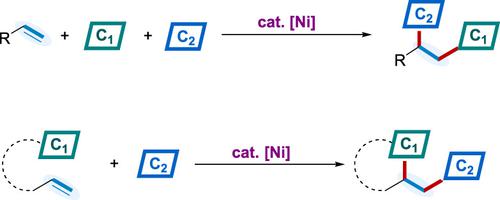
As a straightforward strategy for rapidly increasing molecular complexity, dicarbofunctionalization of alkenes has attracted substantial interests of organic synthesis, medicine chemistry, and materials science. Nickel‐catalyzed cascade dicarbofunctionalizations have been flourished in this area recently, and nickel‐mediated radical pathways particularly offer new opportunities in conjunctive cross‐couplings with alkyl coupling partners. Herein, we give a comprehensive review of nickel‐catalyzed dicarbofunctionalization of alkenes through a historical perspective, including intermolecular three‐component reactions and intramolecular cascade reactions. Among the pathways discussed in this review, the carbometallation/cross‐coupling process and the radical addition/cross‐coupling process are the two major pathways for the nickel‐catalyzed dicarbofunctionalization of alkenes. The oxidative cyclization and 1,2‐metallate shift processes are also selectively discussed. These methods overcome the limitations associated with the reactions using noble metals in the field, providing an efficient and straightforward access to structurally diversified molecules. 
中文翻译:

镍催化烯烃的双碳官能化†
作为迅速增加分子复杂性的直接策略,烯烃的二碳官能化吸引了有机合成,药物化学和材料科学的巨大兴趣。镍催化的级联二碳官能化最近在该领域蓬勃发展,并且镍介导的自由基途径特别为与烷基偶联伙伴进行的交叉偶联提供了新的机会。在此,我们从历史的角度全面回顾了镍催化的烯烃的双碳官能化,包括分子间三组分反应和分子内级联反应。在这篇评论中讨论的途径中,碳金属化/交叉偶联过程和自由基加成/交叉偶联过程是镍催化烯烃双碳官能化的两个主要途径。还选择性地讨论了氧化环化和1,2-金属化物的转变过程。这些方法克服了与使用贵金属的反应在本领域中相关联的局限性,从而提供了对结构多样的分子的有效而直接的途径。
更新日期:2020-06-16

中文翻译:

镍催化烯烃的双碳官能化†
作为迅速增加分子复杂性的直接策略,烯烃的二碳官能化吸引了有机合成,药物化学和材料科学的巨大兴趣。镍催化的级联二碳官能化最近在该领域蓬勃发展,并且镍介导的自由基途径特别为与烷基偶联伙伴进行的交叉偶联提供了新的机会。在此,我们从历史的角度全面回顾了镍催化的烯烃的双碳官能化,包括分子间三组分反应和分子内级联反应。在这篇评论中讨论的途径中,碳金属化/交叉偶联过程和自由基加成/交叉偶联过程是镍催化烯烃双碳官能化的两个主要途径。还选择性地讨论了氧化环化和1,2-金属化物的转变过程。这些方法克服了与使用贵金属的反应在本领域中相关联的局限性,从而提供了对结构多样的分子的有效而直接的途径。












































 京公网安备 11010802027423号
京公网安备 11010802027423号