当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spy chemistry-enabled protein directional immobilization and protein purification.
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-06-16 , DOI: 10.1002/bit.27460 Zhanglin Lin 1 , Qiao Lin 1 , Jiahui Li 1 , Marco Pistolozzi 1 , Lei Zhao 1 , Xiaofeng Yang 1 , Yanrui Ye 1
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-06-16 , DOI: 10.1002/bit.27460 Zhanglin Lin 1 , Qiao Lin 1 , Jiahui Li 1 , Marco Pistolozzi 1 , Lei Zhao 1 , Xiaofeng Yang 1 , Yanrui Ye 1
Affiliation
|
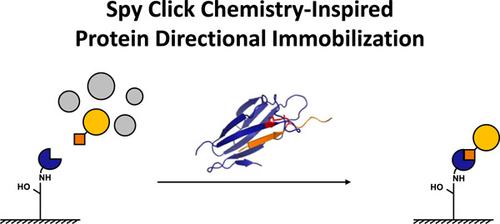
Site‐directed protein immobilization allows the homogeneous orientation of proteins with high retention of activity, which is advantageous for many applications. Here, we report a facile, specific, and efficient strategy based on the SpyTag‐SpyCatcher chemistry. Two SpyTag‐fused model proteins, that is, the monomeric red fluorescent protein (RFP) and the oligomeric glutaryl‐7‐aminocephalosporanic acid acylase, were easily immobilized onto a SpyCatcher‐modified resin directly from cell lysates, with activity recoveries in the range of 85–91%. This strategy was further adapted to protein purification, which proceeded through the selective capture of the SpyCatcher‐fused target proteins by a SpyTag‐modified resin, with the aid of an intein to generate authentic N‐termini. For two model proteins, that is, RFP and a variable domain of a heavy chain antibody, the yields were ∼3–7 mg/L culture with >90% purities. This approach could provide a versatile tool for producing high‐performance immobilized protein devices and proteins for industrial and therapeutic uses.
中文翻译:

间谍化学支持的蛋白质定向固定和蛋白质纯化。
定点蛋白质固定允许蛋白质均匀定向并保持高活性,这对于许多应用来说都是有利的。在这里,我们报告了一种基于 SpyTag-SpyCatcher 化学的简单、具体和有效的策略。两种 SpyTag 融合模型蛋白,即单体红色荧光蛋白 (RFP) 和寡聚戊二酰-7-氨基头孢烷酸酰基转移酶,很容易直接从细胞裂解液中固定到 SpyCatcher 修饰的树脂上,其活性回收率在85–91%。该策略进一步适用于蛋白质纯化,通过 SpyTag 修饰的树脂选择性捕获 SpyCatcher 融合的靶蛋白,并借助内含肽生成真正的 N 末端。对于两种模型蛋白,即 RFP 和重链抗体的可变结构域,产量为 ∼3–7 mg/L 培养物,纯度 >90%。这种方法可以提供一种多功能工具,用于生产用于工业和治疗用途的高性能固定化蛋白质装置和蛋白质。
更新日期:2020-06-16
中文翻译:

间谍化学支持的蛋白质定向固定和蛋白质纯化。
定点蛋白质固定允许蛋白质均匀定向并保持高活性,这对于许多应用来说都是有利的。在这里,我们报告了一种基于 SpyTag-SpyCatcher 化学的简单、具体和有效的策略。两种 SpyTag 融合模型蛋白,即单体红色荧光蛋白 (RFP) 和寡聚戊二酰-7-氨基头孢烷酸酰基转移酶,很容易直接从细胞裂解液中固定到 SpyCatcher 修饰的树脂上,其活性回收率在85–91%。该策略进一步适用于蛋白质纯化,通过 SpyTag 修饰的树脂选择性捕获 SpyCatcher 融合的靶蛋白,并借助内含肽生成真正的 N 末端。对于两种模型蛋白,即 RFP 和重链抗体的可变结构域,产量为 ∼3–7 mg/L 培养物,纯度 >90%。这种方法可以提供一种多功能工具,用于生产用于工业和治疗用途的高性能固定化蛋白质装置和蛋白质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号