当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isothiourea‐Catalyzed Functionalization of Pyrrolyl‐ and Indolylacetic Acid: Enantioselective Synthesis of Dihydropyridinones and One‐pot Synthesis of Pyridinones
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-06-16 , DOI: 10.1002/ajoc.202000290 Shuyue Zhang 1 , Lucas Bacheley 1 , Claire M. Young 1 , Daniel G. Stark 1 , Timothy O'Riordan 2 , Alexandra M. Z. Slawin 1 , Andrew D. Smith 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-06-16 , DOI: 10.1002/ajoc.202000290 Shuyue Zhang 1 , Lucas Bacheley 1 , Claire M. Young 1 , Daniel G. Stark 1 , Timothy O'Riordan 2 , Alexandra M. Z. Slawin 1 , Andrew D. Smith 1
Affiliation
|
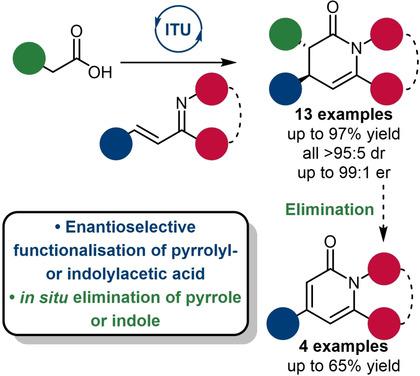
A protocol for the isothiourea‐catalyzed enantioselective functionalization of pyrrolyl‐ and indolylacetic acids has been developed. Stereodefined dihydropyridinones are accessed through formal [4+2] cycloaddition of an in situ generated isothiouronium enolate with α,β‐unsaturated ketimines. The dihydropyridinones are obtained in moderate to excellent yield (26–97%), excellent diastereocontrol (all >95 : 5 dr) and moderate to excellent enantiocontrol (75 : 25–99 : 1 er). An unusual elimination of pyrrole or indole from the dihydropyridinone to access achiral pyridinones was observed and could be selectively promoted. A further one‐pot synthesis using an acyl imidazole was developed to give pyridinones in good to excellent yields (67–91%).
中文翻译:

异硫脲催化的吡咯基和吲哚乙酸的官能化:对映选择性合成二氢吡啶并酮和一锅法合成吡啶并酮
已开发出一种用于异硫脲催化的吡咯基和吲哚基乳酸的对映选择性官能化的方案。立体定义的二氢吡啶酮可通过原位生成的异硫脲铀酸酯与α,β-不饱和酮亚胺的正式[4 + 2]环加成得到。二氢吡啶并酮的得率中等至极好(26-97%),非对映体优异(均> 95:5 dr)和中度至极好对映体(75:25-99:1 er)。观察到从二氢吡啶酮中不寻常地消除了吡咯或吲哚,从而获得了非手性吡啶酮,并且可以选择性地促进这种消灭。还开发了另一种使用酰基咪唑的单锅合成方法,从而使吡啶并酮的收率高至优异(67-91%)。
更新日期:2020-06-16
中文翻译:

异硫脲催化的吡咯基和吲哚乙酸的官能化:对映选择性合成二氢吡啶并酮和一锅法合成吡啶并酮
已开发出一种用于异硫脲催化的吡咯基和吲哚基乳酸的对映选择性官能化的方案。立体定义的二氢吡啶酮可通过原位生成的异硫脲铀酸酯与α,β-不饱和酮亚胺的正式[4 + 2]环加成得到。二氢吡啶并酮的得率中等至极好(26-97%),非对映体优异(均> 95:5 dr)和中度至极好对映体(75:25-99:1 er)。观察到从二氢吡啶酮中不寻常地消除了吡咯或吲哚,从而获得了非手性吡啶酮,并且可以选择性地促进这种消灭。还开发了另一种使用酰基咪唑的单锅合成方法,从而使吡啶并酮的收率高至优异(67-91%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号