Ultrasonics Sonochemistry ( IF 8.7 ) Pub Date : 2020-06-15 , DOI: 10.1016/j.ultsonch.2020.105222 Fatima Zahra Thari 1 , Hamza Tachallait 1 , Nour-Eddine El Alaoui 1 , Aicha Talha 1 , Suhana Arshad 2 , Eleuterio Álvarez 3 , Khalid Karrouchi 4 , Khalid Bougrin 5
|
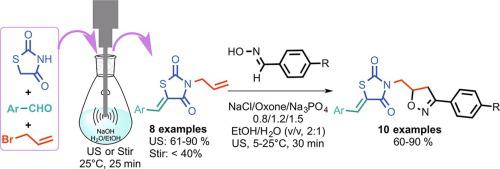
A rapid and green method for the synthesis of novel N-thiazolidine-2,4-dione isoxazoline derivatives 5 from N-allyl-5-arylidenethiazolidine-2,4-diones 3 as dipolarophiles with arylnitrile oxides via 1,3-dipolar cycloaddition reaction. The corresponding N-allyl substituted dipolarophiles were prepared by one-pot method from thiazolidine-2,4-dione with aldehydes using Knoevenagel condensation followed by N-allylation of thiazolidine-2,4-dione in NaOH aqueous solution under sonication. In addition, the isoxazoline derivatives 5 were synthesized by regioselective and chemoselective 1,3-dipolar cycloaddition using inexpensive and mild NaCl/Oxone/Na3PO4 as a Cl source, oxidant and/or catalyst under ultrasonic irradiation in EtOH/H2O (v/v, 2:1) as green solvent. All synthesized products are furnished in good yields in the short reaction time, and then their structures were confirmed by NMR, mass spectrometry and X-ray crystallography analysis.
中文翻译:

在水介质中使用NaCl / Oxone / Na3PO4超声辅助一锅法绿色合成新的N-取代的5-亚芳基-噻唑烷-2,4-二酮-异恶唑啉衍生物。
一种快速,绿色的方法,通过1,3-偶极环加成反应从N-烯丙基-5-芳基萘并噻唑烷-2,4-二酮3与芳基腈氧化物合成新型N-噻唑烷-2,4-二酮异恶唑啉衍生物5。相应Ñ -烯丙基取代dipolarophiles通过制备一锅法从噻唑烷-2,4-二酮使用Knoevenagel缩合的醛,接着Ñ噻唑烷-2,4-二酮在超声处理下的NaOH水溶液的-allylation。另外,异恶唑啉衍生物5通过廉价和温和的NaCl / Oxone / Na 3 PO 4作为氯离子源,氧化剂和/或催化剂在EtOH / H 2 O(v / v,2: 1)作为绿色溶剂。在较短的反应时间内以高收率提供所有合成产物,然后通过NMR,质谱和X射线晶体学分析确认其结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号