Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-06-16 , DOI: 10.1016/j.jcat.2020.06.007 Viviane S. Vaiss , Carla G. Fonseca , Florence P.N. Antunes , Luiz S. Chinelatto Jr. , Sandra S.X. Chiaro , Wladmir F. Souza , Alexandre A. Leitão
|
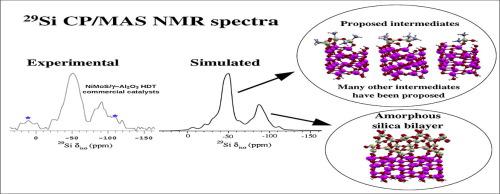
It is known that silicon species originate from the thermal degradation of the polydimethylsiloxane, adsorb on the catalysts surface used for hydrotreatment in the oil refining industry. This severely affects the performance of these catalysts and considerably reduce their lifetime. Combining 29Si and 13C CP/MAS NMR experiments and DFT calculations, we investigated the poisoning process of these catalysts by silicon species in order to obtain a better understanding of this process. Two structural models (mono and bilayer) for amorphous silica deposited on the -Al2O3 surface and some intermediates that can be formed during this process were proposed. From the calculated 29Si chemical shifts, it was possible to identify chemical shifts variations due to different structural elements for each species, thereby, it was possible to make a more appropriate assignment of the resonances found in 29Si CP/MAS NMR spectra in the literature. Besides that, through of the good agreements obtained between the experimental and simulated 29Si CP/MAS NMR spectra, we identified that probably the D4, T1, T3, T4, Q1 and Q2 species are present in the initial stage of the contamination and that the amorphous silica bilayer model deposited on the -Al2O3 surface best characterizes the final stage of the contamination. Moreover, the thermodynamic analysis revealed that the chemisorption reaction of the Hexamethylcyclotrisiloxane (D3) compound is much more stable than the Octamethylcyclotetrasiloxane (D4) compound and of a fragment (corresponding to or of the D3 and D4compounds, respectively), and the chemisorption reaction of the fragment presented the highest values of . This leads us to deduce that the D3 and D4 compounds chemisorb preferentially without breaking. Besides, it also showed that the amorphous silica formation process on the -Al2O3 surface is spontaneous and the bilayer formation reaction is more stable than the monolayer.
中文翻译:

沉积在其表面上的硅物质对失活的HDT催化剂的实验和理论研究:模型命题,结构和热力学分析
已知硅物质源于聚二甲基硅氧烷的热降解,其吸附在炼油工业中用于加氢处理的催化剂表面上。这严重影响了这些催化剂的性能并大大缩短了它们的寿命。结合29 Si和13 C CP / MAS NMR实验和DFT计算,我们研究了硅物种对这些催化剂的中毒过程,以便对该过程有更好的了解。沉积在硅上的无定形二氧化硅的两种结构模型(单层和双层)提出了-Al 2 O 3表面以及在该过程中可能形成的一些中间体。从计算出的29 Si化学位移中,可以确定由于每个物种不同的结构元素而引起的化学位移变化,从而可以更适当地分配29 Si CP / MAS NMR光谱中发现的共振。文学。除此之外,通过实验和模拟的29 Si CP / MAS NMR光谱之间的良好协议,我们确定了D 4,T 1,T 3,T 4,Q 1和Q 2可能是 污染物的初始阶段存在物种,并且无定形二氧化硅双层模型沉积在污染物的初始阶段。 -Al 2 O 3表面最能说明污染的最后阶段。此外,热力学分析表明,六甲基环三硅氧烷(D 3)化合物的化学吸附反应比八甲基环四硅氧烷(D 4)化合物和片段(对应于 要么 分别是D 3和D 4化合物的最大吸附量),并且该片段的化学吸附反应呈现出最高的。这导致我们推断出D 3和D 4化合物优先发生化学吸附而不会断裂。此外,它还表明无定形二氧化硅在硅上的形成过程。-Al 2 O 3表面是自发的,并且双层形成反应比单层稳定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号