Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2020-06-15 , DOI: 10.1016/j.apsb.2020.06.005 Chao Teng 1 , Chenshi Lin 1 , Feifei Huang 1 , Xuyang Xing 1 , Shenyu Chen 1 , Ling Ye 2 , Helena S Azevedo 3 , Chenjie Xu 4 , Zhengfeng Wu 5 , Zhongjian Chen 6 , Wei He 1, 6
|
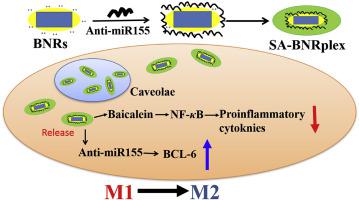
Atherosclerosis (AS) is a lipid-driven chronic inflammatory disease occurring at the arterial subendothelial space. Macrophages play a critical role in the initiation and development of AS. Herein, targeted codelivery of anti-miR 155 and anti-inflammatory baicalein is exploited to polarize macrophages toward M2 phenotype, inhibit inflammation and treat AS. The codelivery system consists of a carrier-free strategy (drug-delivering-drug, DDD), fabricated by loading anti-miR155 on baicalein nanocrystals, named as baicalein nanorods (BNRs), followed by sialic acid coating to target macrophages. The codelivery system, with a diameter of 150 nm, enables efficient intracellular delivery of anti-miR155 and polarizes M1 to M2, while markedly lowers the level of inflammatory factors in vitro and in vivo. In particular, intracellular fate assay reveals that the codelivery system allows for sustained drug release over time after internalization. Moreover, due to prolonged blood circulation and improved accumulation at the AS plaque, the codelivery system significantly alleviates AS in animal model by increasing the artery lumen diameter, reducing blood pressure, promoting M2 polarization, inhibiting secretion of inflammatory factors and decreasing blood lipids. Taken together, the codelivery could potentially be used to treat vascular inflammation.
中文翻译:

抗炎药和抗 miR 155 的细胞内共递送来治疗炎症性疾病。
动脉粥样硬化(AS)是一种发生在动脉内皮下间隙的脂质驱动的慢性炎症性疾病。巨噬细胞在 AS 的发生和发展中发挥着关键作用。在此,利用抗 miR 155 和抗炎黄芩素的靶向共传递将巨噬细胞极化为 M2 表型,抑制炎症并治疗 AS。该共递送系统由无载体策略(药物递送药物,DDD)组成,通过在黄芩素纳米晶体(称为黄芩素纳米棒(BNR))上负载抗miR155,然后用唾液酸包被以靶向巨噬细胞。该共传递系统直径为150 nm,能够有效地在细胞内传递抗miR155并将M1极化为M2,同时显着降低体外和体内炎症因子的水平。特别是,细胞内命运测定表明,共传递系统允许药物在内化后随着时间的推移持续释放。此外,由于延长了血液循环并改善了AS斑块的积聚,共传递系统通过增加动脉管腔直径、降低血压、促进M2极化、抑制炎症因子分泌和降低血脂来显着缓解动物模型中的AS。总而言之,共传递有可能用于治疗血管炎症。











































 京公网安备 11010802027423号
京公网安备 11010802027423号