当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-Selective Copper-Catalyzed Azidation of Benzylic C–H Bonds
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-15 , DOI: 10.1021/jacs.0c05362 Sung-Eun Suh 1 , Si-Jie Chen 1 , Mukunda Mandal 2 , Ilia A Guzei 1 , Christopher J Cramer 2 , Shannon S Stahl 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-15 , DOI: 10.1021/jacs.0c05362 Sung-Eun Suh 1 , Si-Jie Chen 1 , Mukunda Mandal 2 , Ilia A Guzei 1 , Christopher J Cramer 2 , Shannon S Stahl 1
Affiliation
|
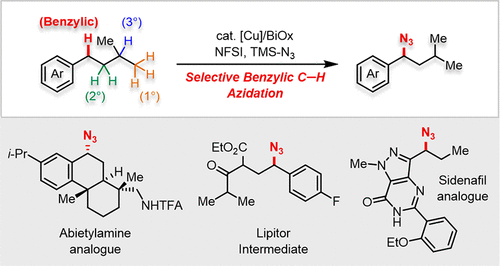
Site selectivity represents a key challenge for non-directed C-H functionalization, even when the C-H bond is intrinsically reactive. Here, we report a copper-catalyzed method for benzylic C-H azidation of diverse molecules. Experimental and density functional theory studies suggest the benzyl radical reacts with a CuII-azide species via a radical-polar crossover pathway. Comparison of this method with other C-H azidation methods highlights the unique site-selectivity of this method, and conversions of the benzyl azide products into amine, triazole, tetrazole, and pyrrole functional groups highlight the broad utility of this method for target molecule synthesis and medicinal chemistry.
中文翻译:

铜催化苄基 C-H 键的位点选择性叠氮化
位点选择性是非定向 CH 官能化的一个关键挑战,即使 CH 键本质上是反应性的。在这里,我们报告了一种铜催化的不同分子的苄基CH叠氮化方法。实验和密度泛函理论研究表明,苄基自由基通过自由基-极性交叉途径与叠氮亚铜物种发生反应。该方法与其他CH叠氮化方法的比较突显了该方法独特的位点选择性,并且将苄基叠氮产物转化为胺、三唑、四唑和吡咯官能团,突显了该方法在目标分子合成和医药方面的广泛用途。化学。
更新日期:2020-06-15
中文翻译:

铜催化苄基 C-H 键的位点选择性叠氮化
位点选择性是非定向 CH 官能化的一个关键挑战,即使 CH 键本质上是反应性的。在这里,我们报告了一种铜催化的不同分子的苄基CH叠氮化方法。实验和密度泛函理论研究表明,苄基自由基通过自由基-极性交叉途径与叠氮亚铜物种发生反应。该方法与其他CH叠氮化方法的比较突显了该方法独特的位点选择性,并且将苄基叠氮产物转化为胺、三唑、四唑和吡咯官能团,突显了该方法在目标分子合成和医药方面的广泛用途。化学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号