当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamics Investigation of Partition Behavior of Uric Acid in Aqueous Two-Phase Systems
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-06-15 , DOI: 10.1021/acs.jced.0c00206 Laryssa Fernanda da Silva Gonçalves 1 , Christian Silva Abreu 1 , Keycianne da Cruz Silva 1 , Aparecida Barbosa Mageste 2 , Guilherme Dias Rodrigues 3 , Wallans Torres Pio dos Santos 4 , Leandro Rodrigues de Lemos 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-06-15 , DOI: 10.1021/acs.jced.0c00206 Laryssa Fernanda da Silva Gonçalves 1 , Christian Silva Abreu 1 , Keycianne da Cruz Silva 1 , Aparecida Barbosa Mageste 2 , Guilherme Dias Rodrigues 3 , Wallans Torres Pio dos Santos 4 , Leandro Rodrigues de Lemos 1
Affiliation
|
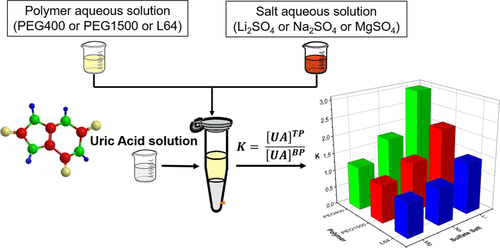
Uric acid (UA) is an important component in biological matrices, and the development of new methods for extracting/separating UA from several complex matrices is necessary. A viable alternative is the use of an aqueous two-phase system (ATPS), which is an environmentally safe and efficient technique. In this work, an extensive study of the thermodynamic approach of UA partitioning was carried out in an ATPS formed with a polymer, sulfate salts, and water. Initially, the new ATPS formed with polyethylene glycol (400 g mol–1), lithium sulfate, and water was characterized by obtaining the position of the binodal curves and the phase compositions. The components’ segregation increases with the increase in the concentration of the polymer and salt where the top phase (TP) becomes richer in polymer and poorer in electrolyte, and the bottom phase has the inverse behavior. In the range of the pH studied, pH 2.40, 5.40, and 6.60 showed no effect on the binodal curve position and phase compositions, while the temperature (288.15, 298.15, and 308.15 K) evaluation indicated that the phase separation process was entropically driven. Afterward, a study of UA partitioning was carried out in several ATPSs, evaluating the effect of system composition, pH, temperature, and ATPS-forming components on the partition coefficient (K) of the UA. The K values ranged from 1.03 ± 0.04 to 6.05 ± 0.25, indicating a partition preference for the TP for all tie-line length (TLL) values. Furthermore, it is noted that the increase in TLL caused an increase in K, which decreases with increasing the temperature; that is, the partition of uric acid is temperature-dependent, and the phase transfer process of the UA is exothermic. The pH effect study showed that the ionized form of UA has a greater interaction with the components of the TP than that of the molecular form because the K value at pH 6.60 (K = 7.59 ± 0.23) is higher than at pH 2.40 (K = 1.98 ± 0.21), while at pH 5.40 (K = 3.84 ± 0.13), the value is intermediate. This behavior is due to the strong electrostatic interaction between the pseudopolycation, formed by Li+ ions plus polyethylene glycol in TP and the ionized form of UA. Finally, higher K values were obtained for the system formed with polyethylene glycol (400 g mol–1), lithium sulfate, and water. Thus, the balance of interactions between the system components and UA is the driving force that will drive the partition in the ATPS.
中文翻译:

两相水体系中尿酸分配行为的热力学研究
尿酸(UA)是生物基质中的重要成分,因此有必要开发新的方法来从多种复杂基质中提取/分离UA。可行的替代方法是使用水相两相系统(ATPS),这是一种对环境安全有效的技术。在这项工作中,在由聚合物,硫酸盐和水形成的ATPS中,对UA分配的热力学方法进行了广泛的研究。最初,新的ATPS与聚乙二醇(400 g mol –1),硫酸锂和水的特征在于获得双曲线曲线的位置和相组成。组分的偏析随着聚合物和盐浓度的增加而增加,其中上层相(TP)变得更富聚合物,而电解质更差,而下相则具有相反的行为。在研究的pH范围内,pH 2.40、5.40和6.60对双曲线曲线的位置和相组成没有影响,而温度(288.15、298.15和308.15 K)评估表明,相分离过程是由熵驱动的。随后,在几种ATPS中进行了UA分配的研究,评估了系统组成,pH,温度和ATPS形成组分对UA分配系数(K)的影响。的K值的范围从1.03±0.04到6.05±0.25,这表示对于所有联系线长度(TLL)值,TP的分区优先级。此外,应注意的是,TLL的增加导致K的增加,而K随温度的升高而降低。也就是说,尿酸的分配与温度有关,UA的相转移过程是放热的。pH值影响研究表明,UA的离子化形式与TP的成分之间的相互作用大于分子形式,因为pH值为6.60(K = 7.59±0.23)的K值高于pH值为2.40(K = pH为5.40(K时为1.98±0.21)= 3.84±0.13),该值为中间值。此行为是由于在TP中由Li +离子加聚乙二醇形成的假聚阳离子与UA的离子化形式之间存在强烈的静电相互作用。最后,由聚乙二醇(400 g mol –1),硫酸锂和水形成的体系获得了更高的K值。因此,系统组件和UA之间交互的平衡是驱动ATPS中分区的驱动力。
更新日期:2020-07-09
中文翻译:

两相水体系中尿酸分配行为的热力学研究
尿酸(UA)是生物基质中的重要成分,因此有必要开发新的方法来从多种复杂基质中提取/分离UA。可行的替代方法是使用水相两相系统(ATPS),这是一种对环境安全有效的技术。在这项工作中,在由聚合物,硫酸盐和水形成的ATPS中,对UA分配的热力学方法进行了广泛的研究。最初,新的ATPS与聚乙二醇(400 g mol –1),硫酸锂和水的特征在于获得双曲线曲线的位置和相组成。组分的偏析随着聚合物和盐浓度的增加而增加,其中上层相(TP)变得更富聚合物,而电解质更差,而下相则具有相反的行为。在研究的pH范围内,pH 2.40、5.40和6.60对双曲线曲线的位置和相组成没有影响,而温度(288.15、298.15和308.15 K)评估表明,相分离过程是由熵驱动的。随后,在几种ATPS中进行了UA分配的研究,评估了系统组成,pH,温度和ATPS形成组分对UA分配系数(K)的影响。的K值的范围从1.03±0.04到6.05±0.25,这表示对于所有联系线长度(TLL)值,TP的分区优先级。此外,应注意的是,TLL的增加导致K的增加,而K随温度的升高而降低。也就是说,尿酸的分配与温度有关,UA的相转移过程是放热的。pH值影响研究表明,UA的离子化形式与TP的成分之间的相互作用大于分子形式,因为pH值为6.60(K = 7.59±0.23)的K值高于pH值为2.40(K = pH为5.40(K时为1.98±0.21)= 3.84±0.13),该值为中间值。此行为是由于在TP中由Li +离子加聚乙二醇形成的假聚阳离子与UA的离子化形式之间存在强烈的静电相互作用。最后,由聚乙二醇(400 g mol –1),硫酸锂和水形成的体系获得了更高的K值。因此,系统组件和UA之间交互的平衡是驱动ATPS中分区的驱动力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号