当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Different Solvent and Conformational Entropy Contributions to the Allosteric Activation and Inhibition Mechanisms of Yeast Chorismate Mutase.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-15 , DOI: 10.1021/acs.biochem.0c00277 Scott D Gorman 1 , Dennis S Winston 1 , Debashish Sahu 1 , David D Boehr 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-15 , DOI: 10.1021/acs.biochem.0c00277 Scott D Gorman 1 , Dennis S Winston 1 , Debashish Sahu 1 , David D Boehr 1
Affiliation
|
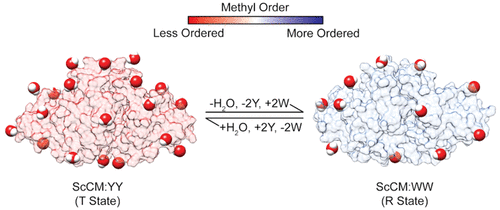
Allosteric regulation is important in many biological processes, including cell signaling, gene regulation, and metabolism. Saccharomyces cerevisiae chorismate mutase (ScCM) is a key homodimeric enzyme in the shikimate pathway responsible for the generation of aromatic amino acids, where it is allosterically inhibited and activated by Tyr and Trp, respectively. Our previous studies indicated that binding of both allosteric effectors is negatively cooperative, that is binding at one allosteric binding site discourages binding at the other, due to the entropic penalty of binding the second allosteric effector. We utilized variable temperature isothermal titration calorimetry (ITC) and nuclear magnetic resonance (NMR) experiments to better understand the entropic contributions to allosteric effector binding, including changes to solvent entropy and protein conformational entropy. Upon binding either Tyr or Trp, ScCM experiences a quenching of motions on the picosecond-to-nanosecond time scale, which we could relate to a loss of protein conformational entropy. Further ITC and NMR studies were consistent with the Tyr-bound form of ScCM being associated with more water molecules compared to the Trp-bound form and Tyr binding being associated with a less positive solvent entropy change. These studies provide insight into the role of structural dynamics in ScCM function and highlight the importance of solvent entropy changes in allosteric regulation, a historically underappreciated concept.
中文翻译:

不同的溶剂和构象熵对酵母水杨酸突变酶的变构活化和抑制机理的贡献。
变构调节在许多生物学过程中都很重要,包括细胞信号传导,基因调节和代谢。酿酒酵母分支酸盐变位酶(ScCM)是the草酸盐途径中的一个重要同型二聚体酶,负责产生芳香族氨基酸,在芳香族氨基酸中它分别被Tyr和Trp变构抑制和激活。我们先前的研究表明,两种变构效应子的结合都是负向合作的,即由于结合第二种变构效应子的熵损失,在一个变构结合位点的结合会阻止在另一个变构结合位点的结合。我们利用可变温度等温滴定热法(ITC)和核磁共振(NMR)实验来更好地了解熵对变构效应子结合的贡献,包括溶剂熵和蛋白质构象熵的变化。绑定Tyr或Trp时,ScCM会经历皮秒到纳秒级的运动猝灭,这可能与蛋白质构象熵的丧失有关。进一步的ITC和NMR研究表明,与Trp结合形式相比,ScCM的Tyr结合形式与更多的水分子相关,而Tyr结合与较少的正溶剂熵变化有关。这些研究提供了对结构动力学在ScCM功能中的作用的洞察力,并强调了溶剂熵变化在变构调节中的重要性,这是历史上未被充分认识的概念。
更新日期:2020-07-14
中文翻译:

不同的溶剂和构象熵对酵母水杨酸突变酶的变构活化和抑制机理的贡献。
变构调节在许多生物学过程中都很重要,包括细胞信号传导,基因调节和代谢。酿酒酵母分支酸盐变位酶(ScCM)是the草酸盐途径中的一个重要同型二聚体酶,负责产生芳香族氨基酸,在芳香族氨基酸中它分别被Tyr和Trp变构抑制和激活。我们先前的研究表明,两种变构效应子的结合都是负向合作的,即由于结合第二种变构效应子的熵损失,在一个变构结合位点的结合会阻止在另一个变构结合位点的结合。我们利用可变温度等温滴定热法(ITC)和核磁共振(NMR)实验来更好地了解熵对变构效应子结合的贡献,包括溶剂熵和蛋白质构象熵的变化。绑定Tyr或Trp时,ScCM会经历皮秒到纳秒级的运动猝灭,这可能与蛋白质构象熵的丧失有关。进一步的ITC和NMR研究表明,与Trp结合形式相比,ScCM的Tyr结合形式与更多的水分子相关,而Tyr结合与较少的正溶剂熵变化有关。这些研究提供了对结构动力学在ScCM功能中的作用的洞察力,并强调了溶剂熵变化在变构调节中的重要性,这是历史上未被充分认识的概念。









































 京公网安备 11010802027423号
京公网安备 11010802027423号