当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Membrane-Dependent Binding and Entry Mechanism of Dopamine into Its Receptor.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-06-15 , DOI: 10.1021/acschemneuro.9b00656 Fabio Lolicato 1, 2 , Hanna Juhola 3 , Agata Zak 4 , Pekka A Postila 5 , Annina Saukko 6, 7 , Sami Rissanen 3 , Giray Enkavi 1 , Ilpo Vattulainen 1, 3, 8 , Mariusz Kepczynski 4 , Tomasz Róg 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-06-15 , DOI: 10.1021/acschemneuro.9b00656 Fabio Lolicato 1, 2 , Hanna Juhola 3 , Agata Zak 4 , Pekka A Postila 5 , Annina Saukko 6, 7 , Sami Rissanen 3 , Giray Enkavi 1 , Ilpo Vattulainen 1, 3, 8 , Mariusz Kepczynski 4 , Tomasz Róg 1
Affiliation
|
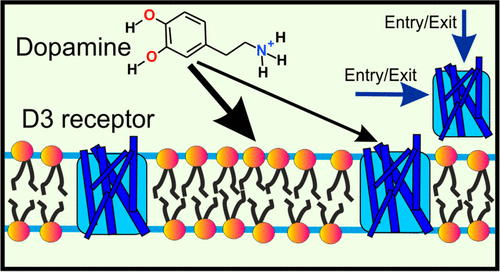
Synaptic neurotransmission has recently been proposed to function via either a membrane-independent or a membrane-dependent mechanism, depending on the neurotransmitter type. In the membrane-dependent mechanism, amphipathic neurotransmitters first partition to the lipid headgroup region and then diffuse along the membrane plane to their membrane-buried receptors. However, to date, this mechanism has not been demonstrated for any neurotransmitter–receptor complex. Here, we combined isothermal calorimetry measurements with a diverse set of molecular dynamics simulation methods to investigate the partitioning of an amphipathic neurotransmitter (dopamine) and the mechanism of its entry into the ligand-binding site. Our results show that the binding of dopamine to its receptor is consistent with the membrane-dependent binding and entry mechanism. Both experimental and simulation results showed that dopamine favors binding to lipid membranes especially in the headgroup region. Moreover, our simulations revealed a ligand-entry pathway from the membrane to the binding site. This pathway passes through a lateral gate between transmembrane alpha-helices 5 and 6 on the membrane-facing side of the protein. All in all, our results demonstrate that dopamine binds to its receptor by a membrane-dependent mechanism, and this is complemented by the more traditional binding mechanism directly through the aqueous phase. The results suggest that the membrane-dependent mechanism is common in other synaptic receptors, too.
中文翻译:

多巴胺的膜依赖性结合和进入机制。
近年来,根据神经递质的类型,已提出突触神经传递通过膜独立或膜依赖机制起作用。在膜依赖性机制中,两亲性神经递质首先分配至脂质头基区域,然后沿膜平面扩散至其膜埋入受体。但是,迄今为止,尚未针对任何神经递质-受体复合物证明这种机制。在这里,我们将等温量热法测量与多种分子动力学模拟方法相结合,以研究两亲性神经递质(多巴胺)的分配及其进入配体结合位点的机制。我们的结果表明,多巴胺与其受体的结合与膜依赖性结合和进入机制一致。实验和模拟结果均表明,多巴胺有利于结合脂质膜,尤其是在头基区域。此外,我们的模拟揭示了从膜到结合位点的配体进入途径。该途径穿过跨膜α-螺旋5和6之间的横向门,该跨膜在蛋白的面向膜侧。总而言之,我们的结果表明,多巴胺通过膜依赖性机制与其受体结合,而这直接通过水相被更传统的结合机制所补充。结果表明,膜依赖性机制在其他突触受体中也很常见。我们的模拟揭示了从膜到结合位点的配体进入途径。该途径穿过蛋白膜表面一侧跨膜α-螺旋5和6之间的侧向门。总而言之,我们的结果表明,多巴胺通过膜依赖性机制与其受体结合,而这直接通过水相被更传统的结合机制所补充。结果表明,膜依赖性机制在其他突触受体中也很常见。我们的模拟揭示了从膜到结合位点的配体进入途径。该途径穿过蛋白膜表面一侧跨膜α-螺旋5和6之间的侧向门。总而言之,我们的结果表明,多巴胺通过膜依赖性机制与其受体结合,而这直接通过水相被更传统的结合机制所补充。结果表明,膜依赖性机制在其他突触受体中也很常见。并通过直接通过水相的更传统的结合机理加以补充。结果表明,膜依赖性机制在其他突触受体中也很常见。并通过直接通过水相的更传统的结合机理加以补充。结果表明,膜依赖性机制在其他突触受体中也很常见。
更新日期:2020-07-01
中文翻译:

多巴胺的膜依赖性结合和进入机制。
近年来,根据神经递质的类型,已提出突触神经传递通过膜独立或膜依赖机制起作用。在膜依赖性机制中,两亲性神经递质首先分配至脂质头基区域,然后沿膜平面扩散至其膜埋入受体。但是,迄今为止,尚未针对任何神经递质-受体复合物证明这种机制。在这里,我们将等温量热法测量与多种分子动力学模拟方法相结合,以研究两亲性神经递质(多巴胺)的分配及其进入配体结合位点的机制。我们的结果表明,多巴胺与其受体的结合与膜依赖性结合和进入机制一致。实验和模拟结果均表明,多巴胺有利于结合脂质膜,尤其是在头基区域。此外,我们的模拟揭示了从膜到结合位点的配体进入途径。该途径穿过跨膜α-螺旋5和6之间的横向门,该跨膜在蛋白的面向膜侧。总而言之,我们的结果表明,多巴胺通过膜依赖性机制与其受体结合,而这直接通过水相被更传统的结合机制所补充。结果表明,膜依赖性机制在其他突触受体中也很常见。我们的模拟揭示了从膜到结合位点的配体进入途径。该途径穿过蛋白膜表面一侧跨膜α-螺旋5和6之间的侧向门。总而言之,我们的结果表明,多巴胺通过膜依赖性机制与其受体结合,而这直接通过水相被更传统的结合机制所补充。结果表明,膜依赖性机制在其他突触受体中也很常见。我们的模拟揭示了从膜到结合位点的配体进入途径。该途径穿过蛋白膜表面一侧跨膜α-螺旋5和6之间的侧向门。总而言之,我们的结果表明,多巴胺通过膜依赖性机制与其受体结合,而这直接通过水相被更传统的结合机制所补充。结果表明,膜依赖性机制在其他突触受体中也很常见。并通过直接通过水相的更传统的结合机理加以补充。结果表明,膜依赖性机制在其他突触受体中也很常见。并通过直接通过水相的更传统的结合机理加以补充。结果表明,膜依赖性机制在其他突触受体中也很常见。











































 京公网安备 11010802027423号
京公网安备 11010802027423号