当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantiomeric separation and quantification of R/S-amphetamine in serum using semi-automated liquid-liquid extraction and ultra-high performance supercritical fluid chromatography-tandem mass spectrometry.
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-07-16 , DOI: 10.1002/dta.2879 Hilde Havnen 1 , Miriam Hansen 1 , Olav Spigset 1, 2 , Solfrid Hegstad 1
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-07-16 , DOI: 10.1002/dta.2879 Hilde Havnen 1 , Miriam Hansen 1 , Olav Spigset 1, 2 , Solfrid Hegstad 1
Affiliation
|
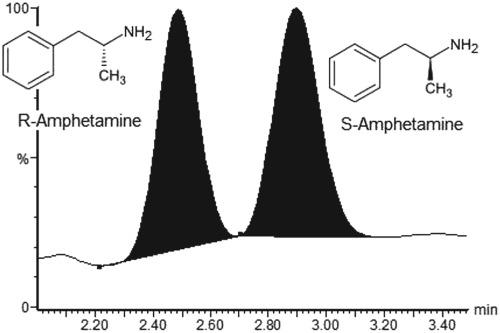
The amphetamine molecule contains a chiral center and its enantiomers exhibit differences in pharmacological effects, with the S‐enantiomer mediating most of the central nervous system stimulating activity. The majority of prescribed amphetamine consists of the pure S‐enantiomer, but therapeutic formulations containing the R‐enantiomer in various proportions are also available. Illegal amphetamine remains available mainly as a racemic mixture of the R‐ and S‐enantiomers. To distinguish between legal and illegal consumption of amphetamine a method for enantiomeric separation and quantification of R/S‐amphetamine in serum was developed and validated using ultra‐high performance supercritical fluid chromatography‐tandem mass spectrometry (UHPSFC‐MS/MS). Sample preparation prior to UHPSFC‐MS/MS analysis was performed by a semi‐automated liquid–liquid extraction method. The UHPSFC‐MS/MS method used a Chiralpak AD‐3 column with a mobile phase consisting of CO2 and 0.1% ammonium hydroxide in 2‐propanol/methanol (50/50, v/v). The injection volume was 2 μL and run time was 4 minutes. MS/MS detection was performed with positive electrospray ionization and two multiple reaction monitoring transitions (m/z 136.1 > 119.0 and m/z 136.1 > 91.0). The calibration range was 12.5–1,000 nM for each analyte. The between‐assay relative standard deviations were in the range of 1.3–3.0%. Recovery was 73% and matrix effects ranged from 95 to 100% when corrected with internal standard. After development and validation, the method has been successfully implemented in our laboratory for both separation and quantification of R/S‐amphetamine and has proved to be a reliable and useful tool for distinguishing intake of R‐ and S‐amphetamine in authentic patient samples.
中文翻译:

使用半自动液-液萃取和超高效超临界流体色谱-串联质谱法对血清中的 R/S-苯丙胺进行对映体分离和定量。
苯丙胺分子包含一个手性中心,其对映异构体在药理作用上表现出差异,S-对映异构体介导了大部分中枢神经系统刺激活性。大多数处方安非他明由纯 S-对映异构体组成,但也有包含不同比例的 R-对映异构体的治疗制剂。非法苯丙胺仍然主要以 R 和 S 对映异构体的外消旋混合物形式提供。为了区分苯丙胺的合法和非法消费,开发了一种对血清中 R/S-苯丙胺进行对映体分离和定量的方法,并使用超高效超临界流体色谱-串联质谱 (UHPSFC-MS/MS) 进行了验证。UHPSFC-MS/MS 分析前的样品制备是通过半自动液-液萃取方法进行的。UHPSFC-MS/MS 方法使用 Chiralpak AD-3 色谱柱,流动相由 CO 组成2 % 和 0.1% 氢氧化铵的 2-丙醇/甲醇溶液 (50/50, v/v)。进样体积为 2 μL,运行时间为 4 分钟。MS/MS 检测采用正电喷雾电离和两个多反应监测跃迁(m/z 136.1 > 119.0 和m/z136.1 > 91.0)。每种分析物的校准范围为 12.5–1,000 nM。测定间相对标准偏差在 1.3-3.0% 的范围内。使用内标校正后,回收率为 73%,基质效应范围为 95% 至 100%。经过开发和验证,该方法已在我们实验室成功实施,用于分离和定量 R/S-苯丙胺,并被证明是区分真实患者样本中 R- 和 S-苯丙胺摄入量的可靠且有用的工具。
更新日期:2020-07-16
中文翻译:

使用半自动液-液萃取和超高效超临界流体色谱-串联质谱法对血清中的 R/S-苯丙胺进行对映体分离和定量。
苯丙胺分子包含一个手性中心,其对映异构体在药理作用上表现出差异,S-对映异构体介导了大部分中枢神经系统刺激活性。大多数处方安非他明由纯 S-对映异构体组成,但也有包含不同比例的 R-对映异构体的治疗制剂。非法苯丙胺仍然主要以 R 和 S 对映异构体的外消旋混合物形式提供。为了区分苯丙胺的合法和非法消费,开发了一种对血清中 R/S-苯丙胺进行对映体分离和定量的方法,并使用超高效超临界流体色谱-串联质谱 (UHPSFC-MS/MS) 进行了验证。UHPSFC-MS/MS 分析前的样品制备是通过半自动液-液萃取方法进行的。UHPSFC-MS/MS 方法使用 Chiralpak AD-3 色谱柱,流动相由 CO 组成2 % 和 0.1% 氢氧化铵的 2-丙醇/甲醇溶液 (50/50, v/v)。进样体积为 2 μL,运行时间为 4 分钟。MS/MS 检测采用正电喷雾电离和两个多反应监测跃迁(m/z 136.1 > 119.0 和m/z136.1 > 91.0)。每种分析物的校准范围为 12.5–1,000 nM。测定间相对标准偏差在 1.3-3.0% 的范围内。使用内标校正后,回收率为 73%,基质效应范围为 95% 至 100%。经过开发和验证,该方法已在我们实验室成功实施,用于分离和定量 R/S-苯丙胺,并被证明是区分真实患者样本中 R- 和 S-苯丙胺摄入量的可靠且有用的工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号