当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unexpected Electronic Behavior of Organic Azide and Metal‐Carbyne in Their 1,3‐Dipolar Cycloaddition Reaction
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-06-15 , DOI: 10.1002/cjoc.202000216 Jing‐Xuan Zhang 1 , Fu Kit Sheong 1, 2 , Zhengyu Lu 3 , Hong Zhang 3 , Zhenyang Lin 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-06-15 , DOI: 10.1002/cjoc.202000216 Jing‐Xuan Zhang 1 , Fu Kit Sheong 1, 2 , Zhengyu Lu 3 , Hong Zhang 3 , Zhenyang Lin 1
Affiliation
|
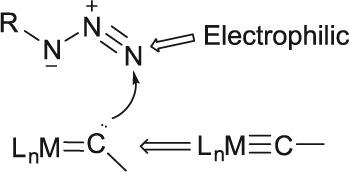
1,3‐Dipolar cycloaddition reaction between organic azide and metal carbyne is a useful strategy to construct metallacycles. However, the electronic behavior of organic azide in 1,3‐dipolar cycloaddition reactions is less explored. This work revealed the unexpected role of organic azide as electrophiles in its cycloadditions with various metal carbynes. The feasibility and regioselectivity of a Fischer‐type osmium carbyne in its reaction with an organic azide are explained by the increased nucleophilicity of carbyne carbon upon bending.
中文翻译:

有机叠氮化物和金属碳烯在1,3-偶极环加成反应中的意外电子行为
有机叠氮化物和金属碳炔之间的1,3-偶极环加成反应是构建金属环的有用策略。但是,有机叠氮化物在1,3-偶极环加成反应中的电子行为很少被探索。这项工作揭示了有机叠氮化物作为亲电子试剂在与各种金属卡宾的环加成中的出乎意料的作用。菲舍尔型碳炔与有机叠氮化物反应的可行性和区域选择性是由于碳炔碳在弯曲时的亲核性提高而解释的。
更新日期:2020-06-15
中文翻译:

有机叠氮化物和金属碳烯在1,3-偶极环加成反应中的意外电子行为
有机叠氮化物和金属碳炔之间的1,3-偶极环加成反应是构建金属环的有用策略。但是,有机叠氮化物在1,3-偶极环加成反应中的电子行为很少被探索。这项工作揭示了有机叠氮化物作为亲电子试剂在与各种金属卡宾的环加成中的出乎意料的作用。菲舍尔型碳炔与有机叠氮化物反应的可行性和区域选择性是由于碳炔碳在弯曲时的亲核性提高而解释的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号