当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aryl Diazonium Salt‐Triggered Cyclization and Cycloaddition Reactions: Past, Present, and Future
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-06-15 , DOI: 10.1002/cjoc.202000270 Fa‐Guang Zhang 1, 2 , Zhen Chen 1 , Chi Wai Cheung 1, 2 , Jun‐An Ma 1, 2
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-06-15 , DOI: 10.1002/cjoc.202000270 Fa‐Guang Zhang 1, 2 , Zhen Chen 1 , Chi Wai Cheung 1, 2 , Jun‐An Ma 1, 2
Affiliation
|
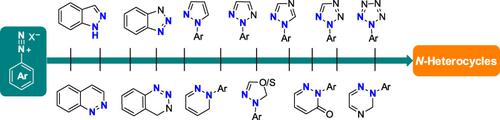
Aryl diazonium salts occupy a privileged role in synthetic chemistry owing to their ready availability and versatile reactivity. While their applications in accessing diversely functionalized arene derivatives via denitrogenation‐coupling and reduction/addition reactions have been well recognized by practitioners in both academia and industry, recent renaissance in chemical transformations of retaining the key N2‐unit has emerged as a powerful technique to construct various N‐heterocycles. This review covers the history and latest advances in cyclization and cycloaddition reactions using aryl diazonium salts as N2‐annulation synthons. The scope, applications, and opportunities in exploring new chemical space by this sustainable strategy are summarized and discussed.
中文翻译:

芳基重氮盐引发的环化和环加成反应:过去,现在和将来
芳基重氮盐由于易于获得且具有多种反应性,因此在合成化学中占有重要的地位。尽管它们在脱氮偶联和还原/加成反应中获得各种功能化的芳烃衍生物的应用已为学术界和工业界人士所公认,但近来在化学转化中保留关键N 2单元的复兴已成为一种强大的技术,构造各种N杂环。这篇综述涵盖了使用芳基重氮盐作为N 2进行环化和环加成反应的历史和最新进展环合成子。总结和讨论了通过这种可持续发展战略探索新化学空间的范围,应用和机会。
更新日期:2020-06-15

中文翻译:

芳基重氮盐引发的环化和环加成反应:过去,现在和将来
芳基重氮盐由于易于获得且具有多种反应性,因此在合成化学中占有重要的地位。尽管它们在脱氮偶联和还原/加成反应中获得各种功能化的芳烃衍生物的应用已为学术界和工业界人士所公认,但近来在化学转化中保留关键N 2单元的复兴已成为一种强大的技术,构造各种N杂环。这篇综述涵盖了使用芳基重氮盐作为N 2进行环化和环加成反应的历史和最新进展环合成子。总结和讨论了通过这种可持续发展战略探索新化学空间的范围,应用和机会。










































 京公网安备 11010802027423号
京公网安备 11010802027423号