Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.1 ) Pub Date : 2020-06-13 , DOI: 10.1016/j.jphotochem.2020.112696 Yuta Tsubonouchi , Tatsuya Eo , Junichiro Honta , Taisei Sato , Eman A. Mohamed , Zaki N. Zahran , Kenji Saito , Tatsuto Yui , Masayuki Yagi
|
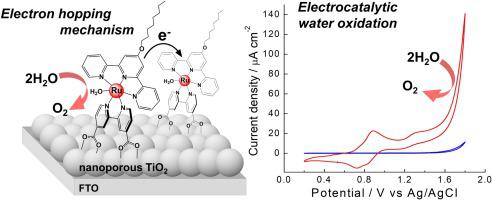
A mononuclear Ru aquo complex, [Ru(C8Otpy)(H2dcbpy)(OH2)]2+ (C8Otpy = 4′-octyloxy-2,2′:6′,2′′-terpyridine, H2dcbpy = 4,4′-dicarboxy-2,2′-bipyridine) was stably adsorbed on a nanoporous TiO2 surface to afford a TiO2 electrode anchoring the complex. The adsorption isothermal of the complex on the TiO2 surface was analyzed by the Langmuir adsorption model to provide a maximum coverage of Гmax = 4.4 × 10−8 mol cm−2 and an adsorption equilibrium constant of Kads = 1.1 × 104 M−1, suggesting that the monolayer of the complex is formed on the TiO2 surface. The cyclic voltammetry measurement of the complex on the TiO2 surface suggested the diffusion-controlled charge transport via electron hopping between the complexes through the TiO2 layer. Pourbaix diagram showed that the complex undergoes a non-H+-coupled 1e- redox reaction of a RuIIOH/RuIIIOH pair of the complex on the TiO2 electrode due to pH buffering ability of the TiO2 surface in a range of pH 5−11. Bulk electrolysis at 1.7 V vs Ag/AgCl using the complex-anchoring TiO2 electrode provided a high and steady catalytic current density of 0.39 mA cm−2 with 83 % Faradaic efficiency for O2 evolution during 1 h electrolysis. However, we presume that the complex is transformed to RuOx nanoparticles on the TiO2 electrode during the electrocatalysis, on the basis of our earlier report on the complex / mesoporous ITO electrode system (Dalton Trans., 2020, 49, 1416−1423.). The RuOH/TiO2 electrode is the efficient anode for water oxidation under the acidic conditions, irrespective of the molecular catalyst of RuOH and the alternative catalysts formed via its oxidative transformation.
中文翻译:

锚固单核钌(II)水配合物的纳米多孔TiO 2电极的分子特性,电化学性质和水氧化催化
单核钌水络合物[Ru(C 8 Otpy)(H 2 dcbpy)(OH 2)] 2+(C 8 Otpy = 4'-辛氧基-2,2':6',2''-叔吡啶,H将2 dcbpy = 4,4'-二羧基-2,2'-联吡啶稳定地吸附在纳米多孔TiO 2表面上,从而得到固定复合物的TiO 2电极。复在TiO上的吸附等温2表面通过Langmuir吸附模型分析,以提供最大覆盖Г最大 = 4.4×10 -8摩尔厘米-2和的吸附平衡常数ķ广告 = 1.1×10在图4M -1中,表明在TiO 2表面上形成了络合物的单层。在TiO 2表面上对络合物进行的循环伏安法测量表明,络合物之间的电子跳跃通过TiO 2层,通过电子跳跃实现了扩散控制的电荷传输。Pourbaix图显示,由于TiO 2表面在一定范围内的pH缓冲能力,该络合物在TiO 2电极上经历了络合物的Ru II OH / Ru III OH对的非H +偶联的1e-氧化还原反应。 pH 5-11。1.7 V的本体电解vs使用固定复合物的TiO 2电极的Ag / AgCl提供了0.39 mA cm -2的高且稳定的催化电流密度,在1 h电解中析出O 2的法拉第效率为83%。然而,我们推测该复合物转化成的RuO X上的TiO 2纳米颗粒2的电催化过程中电极,该复合物/孔ITO电极系统上(我们较早的报告的基础上道尔顿横贯。,2020,49,1416年至1423年。 )。所述RuOH /的TiO 2电极是用于在酸性条件下的水氧化的有效阳极,而不论分子催化剂的RuOH以及通过其氧化转化形成的替代催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号